


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 9-5-2016
Date: 29-8-2017
Date: 18-9-2017
|
DEHYDROGENATION OF PROPANE (Propene Production)
The catalytic dehydrogenation of propane is a selective reaction that produces mainly propene:
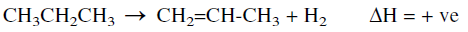
The process could also be used to dehydrogenate butane, isobutane, or mixed LPG feeds. It is a single-stage system operating at a temperature range of 540–680°C and 5–20 absolute pressures. Conversions in the range of 55–65% are attainable, and selectivities may reach up to 95%. Figure 1.1 shows the Lummus-Crest Catofin dehydrogenation process.
For a given dehydrogenation system, i.e., operating temperature and pressure, thermodynamic theory provides a limit to the per pass conversion that can be achieved. A general formula is
Kp = X2P/ (I-X2)
Kp = equilibrium constant at a given temperature
X = fraction paraffin converted to mono-olefins
P = reaction pressure in atmospheres
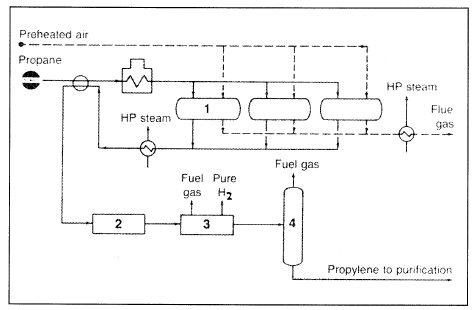
Figure 1.1. The Lummus Crest Catofin dehydrogenation process:3 (1) reactor, (2) compressor, (3) liquid product recovery, (4) product purification.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|