


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 10-8-2018
Date: 7-8-2019
Date: 19-8-2019
|
To understand why some compounds are colored and others are not, and to determine the relationship of conjugation to color, we must make accurate measurements of light absorption at different wavelengths in and near the visible part of the spectrum. Commercial optical spectrometers enable such experiments to be conducted with ease, and usually survey both the near ultraviolet and visible portions of the spectrum.
The visible region of the spectrum comprises photon energies of 36 to 72 kcal/mole, and the near ultraviolet region, out to 200 nm, extends this energy range to 143 kcal/mole. Ultraviolet radiation having wavelengths less than 200 nm is difficult to handle, and is seldom used as a routine tool for structural analysis.
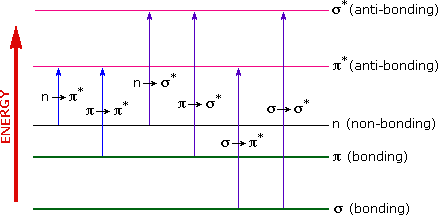
The energies noted above are sufficient to promote or excite a molecular electron to a higher energy orbital. Consequently, absorption spectroscopy carried out in this region is sometimes called "electronic spectroscopy". A diagram showing the various kinds of electronic excitation that may occur in organic molecules is shown on the left. Of the six transitions outlined, only the two lowest energy ones (left-most, colored blue) are achieved by the energies available in the 200 to 800 nm spectrum. As a rule, energetically favored electron promotion will be from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO), and the resulting species is called an excited state.
When sample molecules are exposed to light having an energy that matches a possible electronic transition within the molecule, some of the light energy will be absorbed as the electron is promoted to a higher energy orbital. An optical spectrometer records the wavelengths at which absorption occurs, together with the degree of absorption at each wavelength. The resulting spectrum is presented as a graph of absorbance (A) versus wavelength, as in the isoprene spectrum shown below. Since isoprene is colorless, it does not absorb in the visible part of the spectrum and this region is not displayed on the graph. Absorbance usually ranges from 0 (no absorption) to 2 (99% absorption), and is precisely defined in context with spectrometer operation.
Because the absorbance of a sample will be proportional to the number of absorbing molecules in the spectrometer light beam (e.g. their molar concentration in the sample tube), it is necessary to correct the absorbance value for this and other operational factors if the spectra of different compounds are to be compared in a meaningful way. The corrected absorption value is called "molar absorptivity", and is particularly useful when comparing the spectra of different compounds and determining the relative strength of light absorbing functions (chromophores). Molar absorptivity (ε) is defined as:
|
Molar Absorptivity, ε = A / c l |
(where A= absorbance, c = sample concentration in moles/liter & l = length of light path through the sample in cm.) |
|---|
If the isoprene spectrum on the right was obtained from a dilute hexane solution (c = 4 * 10-5 moles per liter) in a 1 cm sample cuvette, a simple calculation using the above formula indicates a molar absorptivity of 20,000 at the maximum absorption wavelength. Indeed the entire vertical absorbance scale may be changed to a molar absorptivity scale once this information about the sample is in hand. Clicking on the spectrum will display this change in units.
|
Chromophore |
Example |
Excitation |
λmax, nm |
ε |
Solvent |
|
C=C |
Ethene |
π __> π* |
171 |
15,000 |
hexane |
|
C≡C |
1-Hexyne |
π __> π* |
180 |
10,000 |
hexane |
|
C=O |
Ethanal |
n __> π* |
290 |
15 |
hexane |
|
N=O |
Nitromethane |
n __> π* |
275 |
17 |
ethanol |
|
C-X X=Br |
Methyl bromide |
n __> σ* |
205 |
200 |
hexane |
From the chart above it should be clear that the only molecular moieties likely to absorb light in the 200 to 800 nm region are pi-electron functions and hetero atoms having non-bonding valence-shell electron pairs. Such light absorbing groups are referred to as chromophores. A list of some simple chromophores and their light absorption characteristics is provided on the left above. The oxygen non-bonding electrons in alcohols and ethers do not give rise to absorption above 160 nm. Consequently, pure alcohol and ether solvents may be used for spectroscopic studies.
The presence of chromophores in a molecule is best documented by UV-Visible spectroscopy, but the failure of most instruments to provide absorption data for wavelengths below 200 nm makes the detection of isolated chromophores problematic. Fortunately, conjugation generally moves the absorption maxima to longer wavelengths, as in the case of isoprene, so conjugation becomes the major structural feature identified by this technique.
Molar absorptivities may be very large for strongly absorbing chromophores (>10,000) and very small if absorption is weak (10 to 100). The magnitude ofε reflects both the size of the chromophore and the probability that light of a given wavelength will be absorbed when it strikes the chromophore.



|
|
|
|
للحفاظ على صحة العين.. تناول هذا النوع من المكسرات
|
|
|
|
|
|
|
COP29.. رئيس الإمارات يؤكد أهمية تسريع العمل المناخي
|
|
|
|
|
|
|
الامين العام للعتبة الحسينية يؤكد على هيئة التعليم التقني بتحقيق التنمية المستدامة في البلاد
|
|
|