


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 9-11-2016
Date: 19-10-2016
Date: 1-11-2016
|
Photosynthesis
As the name implies, photosynthesis is a process that uses light energy to synthesize something. The term is so general that it could be applied to many different types of reactions, but whenever a botanist uses the term "photosynthesis," the reaction tang discussed is the combination of carbon dioxide with water to form carbohydrate (Fig1). Think about why these particular compounds are part of photosynthesis. First, both carbon dioxide and water are abundant and cheap, occurring almost everywhere in large quantities. The exception is the lack of water in severe deserts like the Sahara, where very Me life exists simply because water is scarce. It is important to have a metabolism based on abundant compounds. It is necessary also for raw materials to be cheap; that is, the plant nut be able to obtain them without expending much energy. Water and carbon dioxide are excellent because they diffuse into the plants automatically from soil, air, or water.
Another important quality of carbon dioxide and water is that they are very stable and contain little chemical energy, so it is possible to deposit a large amount of energy into them The carbohydrates they form are a good means of storing energy because all reactions beading to carbohydrate breakdown have high energy-of-activation barriers. Despite being energy-rich, carbohydrates are stable and chemically unreactive.
Finally, both the reactants and the products of photosynthesis are nontoxic; it is safe to absorb large quantities of carbon dioxide and water and to store high concentrations of carbohydrates. Many substances that are critical for life are extraordinarily toxic if they become even slightly concentrated; chlorine, sodium, ammonium, and many vitamins are just a few examples.
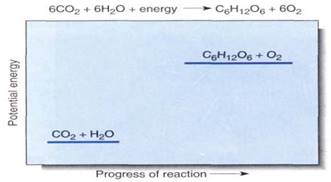
FIGURE 1:Although this chemical equation succinctly summarizes photosynthesis, it reveals virtually nothing of the reaction mechanism or the many carriers and enzymes that participate. We cannot draw a reaction diagram because photosynthesis does not occur by the direct interaction of six molecules of carbon dioxide with six of water; however, the relative potential energies can be shown, indicating that this is an endergonic process.
During photosynthesis, the carbon of carbon dioxide is reduced and energy is supplied to iy, converting it to carbohydrate (Fig. 1). The carbon atom in carbon dioxide is at the +4 oxidation state, while the carbon atoms in carbohydrate are, in general, at the +0 oxidation state. Four electrons must be found and placed into new bonding orbitals around the carbon atom to reduce it. This is not easy, because carbon is more stable in the oxidized safe than in the reduced state: Carbohydrates such as wood and sugar can burn, releasing energy, but carbon dioxide does not. Therefore, a source of electrons and a source of energy are necessary for photosynthesis: The electron source is water and the energy source is light. But water and light do not act on carbon dioxide directly; instead they create the intermediates ATP and NADPH by a process called the light-dependent reactions. In a separate set of reactions, the stroma reactions (formerly known as the dark reactions), ATP and NADPH interact with carbon dioxide and actually produce carbohydrate (Fig. 2)
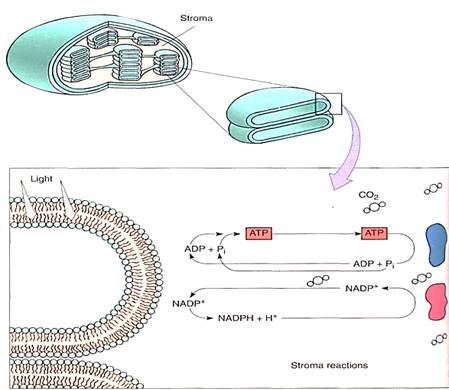
FIGURE 2:Light-dependent reactions of photosynthesis occur by means of membrane-bound carriers, but the actual formation of carbohydrate occurs in the chloroplast liquid (stroma). ATP-ADP and NADP+-NADPH diffuse between the two regions. No region of the chloroplast is far from a membrane, so the distances traveled are only a few hundred times the diameter of a molecule.
THE LIGHT-DEPENDENT REACTIONS
The Nature of Light. Light is one small segment of the electromagnetic radiation spectrum which encompasses gamma rays, X-rays, ultraviolet light, infrared light, microwaves, and radio waves in addition to visible light (Fig. 3). Radiation can be thought of and Sated physically either as a set of particles called quanta (sing. quantum), also called photons, or as a set of waves. The various types of radiation differ from each other only in their wavelengths and the amounts of energy each individual quantum contains. Those radiations with short wavelengths (cosmic rays, gamma rays, ultraviolet light) have relatively large amounts of energy in each quantum, whereas those with long wavelengths (infrared, microwaves, radar, and radio waves) have relatively little. Because humans use visible light for vision (that is why it is visible), we are more sensitive to and familiar with this region of the spectrum. We cannot see other wavelengths, but we can feel infrared radiation as heat, and our bodies react to ultraviolet light by becoming tanned or burned. We distinguish differences in quantum energy (wavelength) of visible light as differences in air Most of us see all wavelengths from red (760 nm) through orange, yellow, green, blue indigo, to violet (390 nm). Certain insects see some near ultraviolet, but in general all animals see in the range from 350 to 760 nm, which is also the radiation tat plants use for photosynthesis.
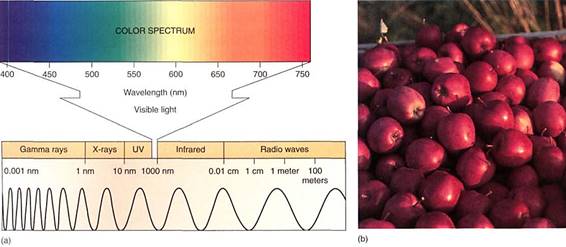
FIGURE 3 :(a) On the left end of this electromagnetic spectrum are gamma rays. If treated as waves, they have extremely short wavelengths, less than 1 nm. If treated as particles, each quantum is highly energetic. Moving to the right, wavelengths become longer and quanta less energetic. The region to which our eyes respond—the visible region—is enlarged. (b) These apples have an abundance of pigments that absorb all colors except red. Earlier, while still developing, they had the pigment chlorophyll which absorbs red and blue strongly, but reflects green.
The Nature of Pigments. Most materials absorb certain wavelengths of light much more than other wavelengths. If a substance absorbs all wavelengths except red, then red light either bounces off or passes through it, and the substance appears red to us. Any material that absorbs certain wavelengths specifically and therefore has distinctive color is a pig- meat but we more often think of pigments as substances that absorb light as part of their biological function. Some pigments, such as melanin (the pigment of suntanned skin), absorb light and thereby protect other light-sensitive substances. Other pigments, such as those in flowers, fruits, or the skins of animals, are important for the light they do not absorb, which gives the pigments their color and allows them to be useful to the organisms in attracting mates, pollinators, or frugivores or in hiding from predators (Fig. 3b).
Photosynthetic pigments transfer absorbed light energy to electrons that then enter chemical reactions. Because only absorbed energy can be used, a theoretically ideal photosynthetic pigment would be black: It would absorb and use all light, not letting any escape. The pigment should at least absorb high-energy radiation (ultraviolet light and gamma rays) instead of the fairly weak visible light. Rather surprisingly, the critical pigment, chlorophyll a, is not like this at all. It absorbs only some red and some blue light, letting most of the rest pass through, especially high-energy radiation. It is important to consider why chlorophyll a does not have what seem like ideal, characteristics.
First, chlorophyll, like all other biological pigments, does not utilize high-energy quanta because they have too much energy. Each is so powerful that it would knock the electrons completely away from the pigment, disrupting bonding orbitals and causing the molecule to break apart. Notice in Figure 10.2 that all bonds in the chlorophyll ring system are double bonds that alternate with single bonds (conjugated double bonds); this bond system is excellent for absorbing quanta, but if even a single electron is knocked out, the whole structure becomes useless. It is selectively disadvantageous for a plant to produce a photosynthetic pigment that is destroyed by the light it absorbs; the molecule would break down and all the ATP that had been expended earlier in its construction would be wasted.
Long wavelength radiations, such as infrared and microwaves, have so little energy par quantum that they cannot appreciably boost an electron's energy. Instead, they make the pigment molecule warmer, as in a microwave oven, but this is not especially useful for chemical synthesis. Visible light contains just the right amount of energy per quantum. When one of these quanta is absorbed by the pigment, an electron is activated—raised to an orbital of a higher energy level. We say that the electron and the molecule go from the ground state to an excited state (Fig. 4). Under the right conditions, this high-energy,
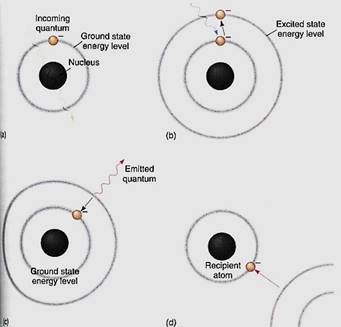
FIGURE 4 (a) If a quantum has the wrong wavelength for a pigment, it passes through the pigment's bonding orbitals without being absorbed. Chlorophyll looks green because most green light passes through it. (b) If the quantum has the correct wavelength, the correct amount of energy, it is absorbed and the electron must move to a new orbital whose energy level corresponds to the electron's new energy load. (c) The excited state is unstable; it may stabilize itself by having the electron emit enough energy (fluoresce) to drop back to its original ground state energy level. (d) The electron can also be stabilized by moving to a more stable orbital on an entirely different atom. This is the critical process in photosynthesis; without this step, life would not exist.
electron can be used in chemical reactions. If it is not used, it returns to its original, stable ground orbital by emitting a new quantum of light, one with less energy and a longer wavelength than the one that it absorbed. The release of light by a pigment is called fluorescence (Fig. 4c).
Two of the most useful pieces of information about a photochemical process are its action spectrum and the absorption spectrum of its pigment (Fig. 5). An absorption spectrum is a graph that shows which wavelengths are most strongly absorbed by a pigment, whereas an action spectrum shows which wavelengths are most effective at powering a photochemical process. To initiate a photochemical process, light must first be absorbed, so the action spectrum of a process must match the absorption spectrum of the pigments responsible. The absorption spectrum of chlorophyll a shows that it absorbs red light (especially 660 nm) and blue light (440 nm) very well and the other wavelengths only slightly. It would be better if it could absorb a greater number of wavelengths, but it simply does not do so. Chlorophyll a is the essential photosynthetic pigment in all plants, algae, and cyanobacteria and has existed unaltered by evolution for about 3 billion years. To put this in perspective, the entire Milky Way Galaxy takes 250 million years to make one rotation about its center, so in 12 full rotations of our galaxy, no alteration in the structure of chlorophyll a has been selectively advantageous.
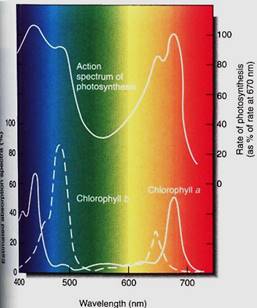
FIGURE 5: The absorption spectra of chlorophyll a and chlorophyll b, and the action spectrum of photosynthesis. On the bottom axis is the wavelength of light, with short (blue) wavelengths to the left and long (red) ones to the right. The vertical axis of the absorption spectra is the amount of light absorbed by the pigment; for the action spectrum, it is the amount of photosynthesis carried out. Chlorophylls absorb little of the very short wavelength light at 400 nm, and little photosynthesis occurs. But light at slightly longer wavelengths, about 425 nm, is absorbed well by chlorophyll a and photosynthesis proceeds. Quanta with intermediate wavelengths pass right through the pigment and photosynthesis is low, but in the 650 to 680 nm range (red) considerable absorption occurs. Because the absorption spectra of chlorophyll a and b are different, more wavelengths can be efficiently harvested. If the two matched perfectly, chlorophyll b would be useless.
Accessory pigments are molecules that strongly absorb wavelengths not absorbed by chlorophyll a. The absorbed energy is passed on to chlorophyll a. In effect, the accessory pigments overcome the narrow absorption of chlorophyll a and broaden the action spectrum of photosynthesis. We know that accessory pigments are involved because the action spectrum of photosynthesis does not perfectly match the absorption spectrum of chlorophyll a. The most common accessory pigments in land plants are chlorophyll b and the carotenoids (Fig. 6); algae have other types. Chlorophyll a and b are large, flat molecules with almost identical porphyrin ring structures. Their phytol tails are hydrophobic and dissolve into the lipid portion of the thylakoid membrane. When packed tightly in a membrane by their phytol tails, their porphyrin rings lie parallel to each other, which causes the electron orbitals of one molecule to interact with those of the two adjacent molecules. Hundreds of chlorophylls act somewhat like one molecule, and the energy absorbed by one can be rapidly transferred to another in a different part of the complex. This transfer, called resonance, allows chlorophyll b to absorb wavelengths that chlorophyll a would miss and then transfer the energy to chlorophyll a for use in chemical reactions. Carotenoids are poor at this type of resonance and transfer only about 10% of their energy; they seem to be more important in absorbing excessive light and thus protecting chlorophylls.

FIGURE 6: (a) There are two types of carotenoids: Carotenes lack oxygen but xanthophylls (such as lutein) have it. Both are accessory pigments that protect the chlorophyll from excess sunlight. (b) Carotenoids are always present in leaves, but usually the abundant chlorophyll masks their presence. In autumn, chlorophyll breaks down and we can see the carotenoids.
Photosystem I. When light excites an electron in chlorophyll a, the electron is so unstable that it either reacts with almost anything or fluoresces, wasting its energy. Chlorophyll molecules and the electron carriers involved in photosynthesis must therefore be tightly bound to the thylakoid membranes of the chloroplast. Chlorophyll's electron must react only with the proper molecule, which should be close enough for the reaction to take place instantly, before fluorescence can occur. All pigments and carriers that work together ate packed into a granule called a photosynthetic unit, and the thylakoid membranes are filled with millions of these granular arrays (Fig. 7). Each photosynethetic unit contains about 300 molecules of chlorophylls a and b and carotenoids. In some photosynthetic units, chlorophyll b is plentiful and in others it is less abundant. Those with little chlorophyll b have been named photosystem I; those in which chlorophyll b is present at levels almost equal to a are photosystem II. The photosystem I units are involved in the Slowing reactions.
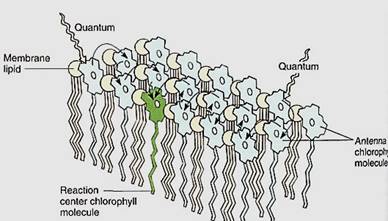
FIGURE 7:Only special chlorophyll a molecules—reaction centers— can actually undergo the initial photochemical reaction of photosynthesis, but they are surrounded by other chlorophyll a molecules as well as accessory pigments; regardless of which pigment absorbs light, the energy is transferred to the reaction center.
When light strikes any pigment of a photosystem I array, either chlorophyll a or an accessory pigment, the energy is transferred to the reaction center, a structure that contains a pair of special molecules of chlorophyll a whose properties differ from all other molecules of chlorophyll a in the unit (Fig. 8). This pair of chlorophylls of the photosystem I reaction center is given the special name P700 because they absorb red light of 700 nm most efficiently. The energy excites an electron of P700, which is then absorbed by a membrane-bound electron acceptor known as "X." This is a transfer of an electron; no bonding orbital is formed. The exact chemical nature of X is not known, but it contains iron and sulfur and is sometimes designated Fe4S4. When X absorbs an electron from P700, it becomes a powerful reducing agent, with a redox potential of —0.73 volts . The transferred electron is still extremely unstable, and the reduced X immediately passes it onto ferredoxin, which is also located in the thylakoid membrane (Fig. 9). Ferredoxin is a small protein (10,500 to 11,000 daltons; a dalton is the weight of one hydrogen atom) with an active site consisting of two iron atoms bound to two sulfur atoms. Reduced ferredoxin is also a strong reducing agent, with a redox potential of —0.43 volts. Electrons are passed from ferredoxin to an enzyme, ferredoxin-NADP+ reductase, which then reduces NADP+, converting it to NADPH, as its name indicates. Ferredoxin carries only one electron, but two are needed simultaneously to reduce NADP+. Ferredoxin-NADP reductase carries two electrons, but it can be reduced one electron at a time; then it transfers those two electrons together to NADP+ . Although NADPH is also a strong reducing agent, it is stable enough to move away from the membrane safely without the risk of reducing things indiscriminantly, as the previous electron carriers might.
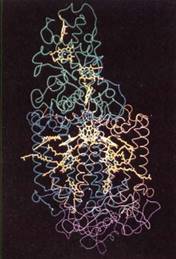
FIGURE 8:Computer model of the reaction center of a photosynthetic bacterium. At the top in green are cytochromes, whose heme groups are yellow. In the middle in blue and brown are proteins with hydrophobic alpha helix secondary structure that fits into the chloroplast membranes. The proteins surround bacteriochlorophylls, carotenoids, and bacteriophaeophytin (yellow). The bottom purple is another protein. Reaction centers in plants have many analogous components that probably have similar positions. (Courtesy of Johann Deisenhofer and Hartmut Michel, University of Texas, Southwestern Medical Center)
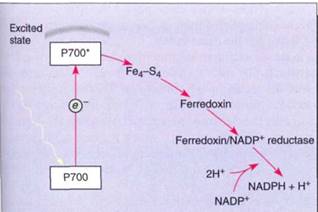
FIGURE 9:In photosystem I, energy is absorbed by a pair of P700 chlorophyll a molecules, raising two electrons to an excited energy level; from here, they pass onto Fe4-S4 ("X"), then onto ferredoxin, and finally onto ferredoxin-NADP reductase. After two electrons have reduced ferredoxin-NADP reductase, they are transferred simultaneously to NADP+, reducing it to NADPH + H+.
Photosystem II. Photosystem I efficiently produces NADPH, but the reaction center P700 chlorophyll a loses electrons during the process. In this oxidized state, bonding orbitals could easily rearrange, causing the molecule to break down and be destroyed. There must be a mechanism that adds electrons back to the P700, reducing it so that it can work repeatedly.
The mechanism that reduces P700 is located in the photosynthetic unit rich in chlorophyll b, photosystem II (Fig. 10). Photosystem II can be best described by working backward from photosystem I: A molecule of plastocyanin, which contains copper, donates an electron to the chlorophyll a of the photosystem I reaction center. The plastocyanin is now oxidized, lacking an electron; it must reacquire one because it also is too expensive a molecule to donate just one electron and then never work again. It receives its new electron from a complex of cytochrome molecules, called the cytochrome b6/f complex, which in turn gets an electron from a molecule of piastoquinone. This receives electrons from another carrier, Q, a molecule of quinone, which in turn receives electrons from phaeophytin. Phaeophytin is actually a chlorophyll a molecule that does not contain a magnesium atom. Phaeophytin becomes oxidized as it donates an electron to Q, so it must obtain another electron, which it does when a chlorophyll a molecule absorbs light and is activated. This is a different chlorophyll a from the one in photosystem I; it is the reaction center of photosystem II and has the name P680.
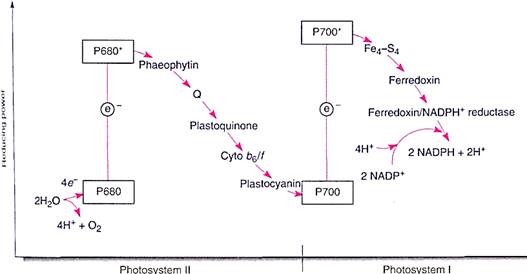
FIGURE 10:The two photosystems work together to transfer electrons from water to NADPH. The scale on the left indicates reducing power, the redox potential (see Table 10.5). The higher a molecule is in the chart, the greater its capacity to force electrons onto another molecule. Because of its shape, this diagram is called a Z scheme.
We may seem to be going in circles: taking an electron from one chlorophyll a, P680, to pass it onto another chlorophyll a, P700, which then sends it to NADP+ . But the physical differences between the two molecules of chlorophyll a are crucial. The one in photosystem II gets new electrons from water, not plastocyanin. The important thing is that water is cheap enough to just throw away after the electrons are removed. Water breaks down into protons (H+), which the plant uses, and oxygen (O2), which it discards. Whereas all electron carriers are large, expensive molecules that the plant must construct itself, water is simply brought in. The electrons are stripped off, the protons are used, and the oxygen is discarded through stomata.
Photosystems I and II together are an efficient system. Electrons are passed from water to P680 in photosystem II, their energy is boosted by light, then they move through an electron transport chain—the various electron carriers—to P700 in photosystem I. Their energy is boosted by light again, and they pass through a short second electron transport chain to NADP+ , reducing it to NADPH. This last step requires that protons be added to NADP+; these protons are present in the water surrounding the membrane (water is always a mixture of H2O, H+ , and OH-). It would be simpler if photosystem I could receive electrons directly from water, but that does not happen. Besides, the electron transport chain between P680 and P700 is necessary for the production of ATP.
The Synthesis of ATP. The light-dependent reactions produce the reducing agent NADPH that actually places electrons onto the carbon of carbon dioxide in the stroma reactions. But the stroma reactions are highly endergonic and must be driven by being coupled to the exergonic splitting of ATP. The necessary ATP is also generated by the light reactions, but the process is indirect. It is photophosphorylation because light is involved, but a more specific name is often used: chemiosmotic phosphorylation. To understand it we must take a closer look at the structure of chloroplasts. As mentioned in Chapter 3, the inner membrane of chloroplasts folds inward, forming flattened sacs called thylakoids (Fig 11). In certain regions, these swell slightly and form rounded vesicles. All thylakoids in one region form vesicles at the same spot, so they occur in sets called grana (singular: granum). Thylakoids that lie between grana are frets. The liquid surrounding the thylakoid system is the stroma, but notice especially that there is another compartment.
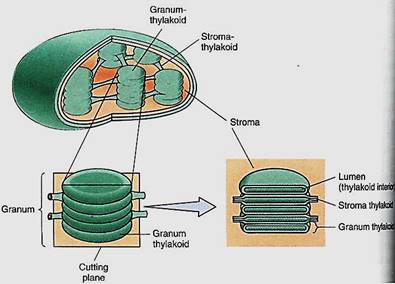
FIGURE 11:Grana are stacks of small thylakoid vesicles compressed together; frets are regions of thylakoid that connect one granum to another. The lumen of the thylakoid region is continuous with that of the fret region. The liquid surrounding all the thylakoids is stroma.
The thylakoid lumen is a critically important compartment because some of the enzymes and electron carriers of the photosystems are embedded in the membrane layer facing the lumen, whereas other enzymes are in the membrane layer that faces the stroma (Fig. 12). The reactions that break down water and produce oxygen and protons are located on the lumen side of the thylakoid membrane, in the granum areas. This membrane is not permeable to protons, so as the light reactions run, the thylakoid interior accumulates protons and their concentration increases. The molecules of ferredoxin-NADP reductase that generate NADPH are located on the other side of the membrane, facing the stroma. The protons they attach to NADP+ are those present as a result of the natural breakdown of wafer ; As the protons are absorbed, their concentration in the stroma decreases. Furthermore, during electron transport between P680 and P700, the electron carrier plastoquinone moves a proton from the stroma to the thylakoid lumen every time it carries an electron between phaeophytin and the cytochrome b6/f complex. This also contributes to the increased concentration of protons in the thylakoid lumen and to the decreased concentration of protons in the stroma.

FIGURE 12:The water-splitting, proton-producing reactions of photosystem II take place on the lumen side of the thylakoid membrane. Plastoquinone is like NADP+ in that when it picks up electrons, it also picks up a proton. This occurs on the stroma side of the membrane, but the reduced plastoquinone must diffuse to the other side of the membrane to pass electrons on to the cytochrome b6/f complex. The proton then dissociates and is deposited in the lumen, adding to the growing pool of protons. When NADPH is formed, it picks up protons from the stroma, so this and the plastoquinone pumping result in a deficiency of protons in the stroma. Protons return to the stroma by passing through ATP synthetases; their passage is exergonic and powers the phosphorylation of ADP to ATP.
The strong difference between the concentrations of protons inside the thylakoid lumen and exterior to it in the stroma quickly becomes so powerful that protons begin to flow out of the lumen through special channels in the membrane. These channels are complex sets of enzymes that can synthesize ATP from ADP and phosphate; the entire complex is called ATP synthetase (Fig. 12). The ATP synthetase of chloroplasts is known specifically as the CF0-CF1 complex. CF0 is the portion of the enzyme spanning the membrane where the actual proton channel is located. CF1 is the portion of the enzyme that phosphorylates ADP to ATP. The power required to force phosphate onto ADP and establish the high-energy bonding orbitals of ATP comes from the flow of protons through the ATP synthetase channels.
When electrons flow smoothly from water to NADPH, the process is called noncyclic electron transport (Figs. 10 and 13). The chemiosmotic potential that builds up does not produce quite enough ATP for the stroma reactions: There is too little ATP relative to ,the amount of NADPH produced. This problem is overcome by an alternate route £r electrons. Once they reach ferredoxin in photosystem I, they can be transferred to the plastoquinones of photosystem II instead of being used to make NADPH. The plastoquinones carry the electrons along just as though they had gotten them from Q and use their energy to pump more protons into the thylakoid lumen. This is cyclic electron transport, and with it chloroplasts make extra ATP without making extra NADPH, thus producing ATP and NADPH in the proper ratios for the stroma reactions. Cyclic electron transport is a simple light-powered proton pump, and similar types occur in bacteria. This may have been the original power system that evolved first, billions of years ago.
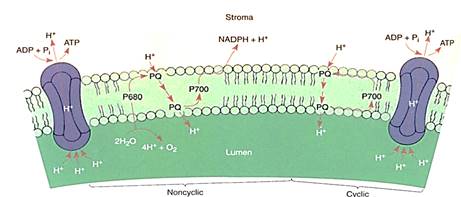
FIGURE 13:In noncyclic electron transport, electrons flow through the Z scheme from water to NADPH. Cyclic electron transport is much simpler: Electrons flow from P700 to piastoquinone, which carries a proton to the lumen and returns the electron to P700.
THE STROMA REACTIONS
The conversion of carbon dioxide to carbohydrate occurs in the stroma reactions, which are also called the Calvin/Benson cycle, or the C3 cycle (Fig. 14). These reactions take place in the stroma, mediated by enzymes that are not bound to thylakoid membranes . In the first step, an acceptor molecule (ribulose-l,5-bisphosphate; RuBp) reacts with a molecule of carbon dioxide. Because RuBP contains five carbons and one more is added from carbon dioxide, you might expect a product that contains six carbons However, the new molecule breaks apart immediately, while still on the enzyme; stable bonding orbitals cannot be formed between all six carbon atoms while so many oxygen atoms are present and pulling electrons to themselves. Instead, orbitals rearrange and two identical molecules are formed that each contains three carbons: 3-phosphoglycerate, hence the name C3 cycle. The abbreviation PGA is often used for 3-phosphogly cerate.

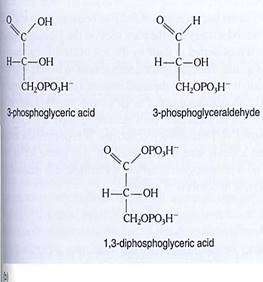
FIGURE 14 (a) In the yellow area are the first steps of the stroma reactions, also known as the C3 cycle; the product is two molecules of 3-phosphoglyceraldehyde. Some of this is transported out of the chloroplast, and the rest undergoes reactions (blue area) that form a new molecule of the acceptor, RuBP. (b) At various times acids such as phosphoglycerate and malate are written as phosphoglyceric acid and malic acid; the "-ic acid" ending refers to the whole acid. The "-ate" ending refers to the acid's anion, the negatively charged portion left after the proton dissociates. In protoplasm, most of the acids occur as free anions, not intact neutral acids still holding their protons.
The enzyme that carries out this reaction has many names; the most common is RBP carboxylase (RUBISCO). This is one of the largest and most complex enzymes known- a giant complex of two kinds of protein subunits. There are eight copies of a small protein, each with a molecular weight of 14,000 to 15,000 daltons, and eight copies of a lag: protein, each with a molecular weight of 53,000 to 55,000 daltons (Fig. 15). The entire enzyme has a molecular weight of about 480,000 daltons. Not only is the tertiary structure of each protein subunit important, but their quaternary structure as a complex is critical.
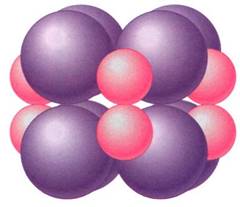
FIGURE 15:RuBP carboxylase. This is a large enzyme in which quaternary structure is critical; the individual proteins have no useful activity until combined in the full complex.
RuBP carboxylase can constitute up to 30% of the protein in a leaf, making it the most abundant protein on Earth. Without it, there would be almost no life at all; all photosynthesis that produces oxygen is mediated by this enzyme. Only a few rare photosynthetic bacteia use a different enzyme. RuBP carboxylase is crucial to the production of food; without it heterotrophs would starve.
Like chlorophyll a, RuBP carboxylase is by no means ideal. Its active site recognizes and binds to carbon dioxide only poorly, and it has low substrate specificity, frequently putting oxygen rather than carbon dioxide onto RuBP. Yet this enzyme is highly conserved evolutionarily: The amino acid sequences of RuBP carboxylase from all plants are virtually identical. Apparently all mutations that cause any change in structure, however slight, dslub the active sites and are selectively disadvantageous.
It is important to realize that the first step of the stroma reactions is a carboxylation only. Electrons and energy are added in the next two steps: ATP donates a high-energy phosphate group to the 3-phosphoglycerate, converting it to 1,3-diphosphoglycerate, which then is reduced by NADPH to 3-phosphoglyceraldehyde (abbreviated PGAL); a phosphate comes off in this step also. The carbon is now both reduced and energized.
The rest of the stroma reactions are complex, but the important point is that as they operate, some 3-phosphoglyceraldehyde can be taken out of the chloroplast and used by the cell to build sugars, fats, amino acids, nucleic acids—basically anything the plant needs. The rest of the 3-phosphoglyceraldehyde remains in the chloroplast and undergoes several more stroma reactions which convert it to RuBP, the original acceptor molecule. The principle involved is important: To incorporate carbon dioxide, the plant needs the acceptor RuBP, and the two react on a one-to-one basis. To assimilate large amounts of carbon dioxide, the plant either needs large amounts of RuBP or needs to use a few REP molecules repeatedly. It is the second strategy that the plant uses. As the 3-phosphoglycer- aldehyde is formed, some of it is reconverted to RuBP by the rest of the stroma reactions and some is exported to the cytoplasm. The chloroplast does not need to import quantities of RuBP from the rest of the cell; it just recycles the small amount that it has. Imagine a chloroplast that has 1,000,000 carbon atoms inside it as the various intermediates of the stroma reactions', after three carbon dioxides have been assimilated there are 1,000,003 carbon atoms. When one molecule of 3-phosphoglyceraldehyde is exported, the carbon pool returns to 1,000,000, and a steady state is maintained. In very young leaves with growing chloroplasts, little or no 3-phosphoglyceraldehyde is exported; it is retained ad the pools of C3 metabolites increase in numbers of molecules.
ANABOLIC METABOLISM
3-Phosphoglyceraldehyde is an amazingly versatile molecule: Using it plus water, nitrates, and minerals, plants construct everything inside themselves. The entire fabric of the organism can be synthesized. This is also the basis of all animal metabolism, because animal either eat plants or eat other animals that eat plants.
Most biological molecules are larger than 3-phosphoglyceraldehyde, so it must be rearranged and altered in the cytoplasm to build up larger, more complex molecules. This constructive metabolism is called anabolism and it consists of anabolic reactions.
Anabolic pathways are numerous, but two are especially important with regard to energy metabolism: the synthetic pathways of polysaccharides and fats, which are sorag2 forms of energy and carbon. The NADPH and ATP produced by photosynthesis are excel-lent sources of energy, but they cannot be stored for even a short time. They are so reactive and unstable that they would break down. A plant cannot stockpile them to survive time when photosynthesis is impossible. Nor can they be transported over long distances, so even if leaves had an abundant supply, roots would starve. Several types of storage compounds have evolved which solve these problems.
1. Short-term storage: ATP and NADPH can be used within the cell and last criy briefly.
2. Intermediate-term storage: The simple sugar glucose and the disaccharide sucrose are stable enough to be moved from cell to cell, either in the vascular tissue of a plant or in a blood stream. They are also sufficiently stable to last for weeks or months. A problem with storing large quantities of mono- or disaccharide is that they cause cells to absorb water by osmosis.
3. Long-term storage: Starch is a large, high-molecular-weight polymer of glucose, too large to be transported. It is even more stable than glucose, lasts for years, and does not cause the cell to absorb water. Lipids are an even more concentrated storage form of energy which can be synthesized rapidly and stored in large quantities.
The Synthesis of Polysaccharides. The anabolic synthesis of glucose is gluconeogenesis (Fig. 16). In reactions similar to those of C3 metabolism, part of the 3-phosphoglyceral- dehyde exported to the cytoplasm is converted to dihydroxyacetone phosphate; one molecule of this condenses with one molecule of unconverted 3-phosphoglyceraldehyde to form the sugar fructose-1,6-bisphosphate. This loses a phosphate to become fructoses- phosphate, and part of this is rearranged, converting it to glucose-6-phosphate. Both fructose-6-phosphate and glucose-6-phosphate are versatile, useful molecules that enter many metabolic pathways. In plants, the glucose-6-phosphate is polymerized into polysaccharides: amylose, amylopectin, or cellulose. It is similarly essential in animals.
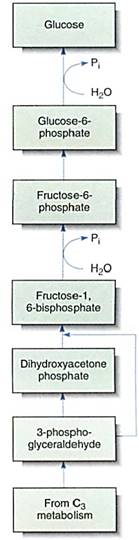
FIGURE 16: Gluconeogenesis is an anabolic pathway in which large molecules are built up from small ones. This process may occur in chloroplasts, amyloplasts, or cytosol.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|