Long Distance Transport: Phloem
Although the exact mechanism by which water and nutrients are moved through phloem is not known, most evidence supports the pressure flow hypothesis. Membrane-bound molecular pumps and active transport are postulated to be the important driving forces.
The sites from which water and nutrients are transported are sources. During spring and summer, leaves are dominant sources as their photosynthetically produced sugars are exported to the rest of the plant. At other times, such as early spring before new leaves have been produced by deciduous trees, the sources are storage sites such as tubers, corms, wood and bark parenchyma, and fleshy taproots. Cotyledons and endosperm are sources for embryos during germination. Within sources, sugars are actively transported into sieve elements—sieve tube members in angiosperms, sieve cells in nonflowering plants. The location of the molecular pumps is not known for certain; it may be the sieve element plasma membrane. But in many species, cells surrounding sieve elements, especially companion cells, appear to be transfer cells of great importance in loading phloem.
As sugars such as sucrose are pumped into sieve elements, the sieve element protoplasm tends to become more concentrated (Fig. 1a). Consequently, both its osmotic potential and its water potential become more negative. This causes hydraulic disequilibrium between the sieve elements and surrounding cells, and water diffuses into the sieve elements. In any other cell, the increased volume of sugars and water would cause the protoplast to expand and press against the cell wall, but sieve elements are unique, being living cells with relatively large holes in the walls, up to 14 µm wide in cucumbers and pumpkins . When pressure starts to build in these cells, "protoplasm" is squeezed through the sieve pores into the next cell. Sieve element protoplasm is not like that of most cells: The vacuolar membrane disintegrates, allowing vacuolar water to mx with part of the cytoplasm, creating an extremely watery, non-viscous substance—the phloem sap. The majority of the protoplasm is held firmly to the walls, probably by microtubules or microfilaments, and is not carried away with the watery central phloem sap.
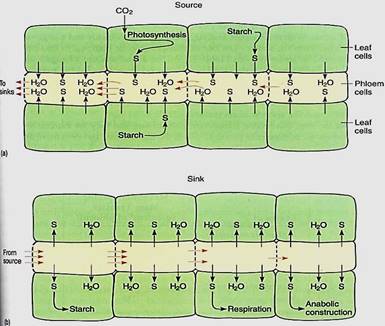
FIGURE 1: (a) As sucrose (s) is actively transported into sieve elements, yp and ycell become more negative, moving away from equilibrium with companion cells and other neighboring cells. Water moves into the sieve elements, squeezing phloem sap out through the sieve pores. Because the sugary water escapes through sieve pores, high pressure does not build up, so the pressure potential does not rise and stop the influx of water. The water potentials of sieve elements and surrounding cells never reach equilibrium as long as sucrose is being pumped. The source of the glucose may be photosynthesis or the breakdown of starch. (b) In sinks, sucrose is actively transported out of sieve elements, and all processes work in reverse compared with sources. In some sinks, most of the sucrose is converted to glucose and then polymerized to starch (storage organs), is respired (most tissues), or is used to construct new compounds (growing tissues).
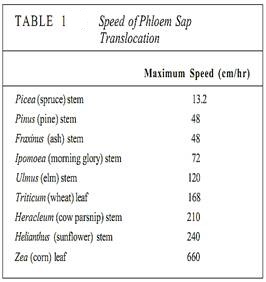
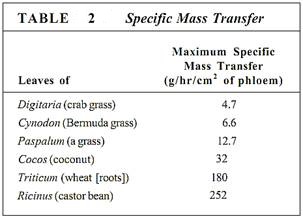
In sources, phloem loading occurs along numerous vascular bundles such as the fine veins in leaves, the network of bundles in tubers and corms, and the inner bark in storage roots and stems. With this massive loading, pressure builds quickly and a large volume of material flows from the source. The rate of transport can be high; up to 660 cm/hr has been measured in leaves of corn (Table 1). The actual amount of sugars and other nutrients (excluding water) transported by phloem per hour is called the mass transfer. Vascular bundles vary not only in the speed at which their phloem translocates but also in the amount of phloem present. The number of bundles leaving a source is also important. To make comparisons easier, mass transfer can be divided by the cross-sectional area of phloem to obtain the specific mass transfer (Table 2).
Sinks are sites that receive transported phloem sap, and they are extremely divese Storage organs are important in perennial plants during summer, but even more important are meristems, root tips, leaf primordia, growing flowers, fruits, and seeds. On even a small tree there may be thousands of sinks, each receiving nutrients. Not all sinks are active simultaneously; most plants do not produce flowers and leaves at the same time, and fruits can develop only after flowers. Within sinks, sugars are actively transported out of sieve elements into surrounding cells. The loss of sugar causes phloem sap to be more dilute, and its osmotic potential and water potential tend to become less negative, so water diffuses outward into the surrounding cells (Fig. 1b). As a result, even though phloem sap flows rapidly into a sink, the end cells of the phloem do not swell. As rapidly as nutrients are loaded at sources, they are unloaded at sinks.
Because phloem sap is under pressure, the danger exists of uncontrolled "bleeding" if the phloem is cut. Vascular bundles are broken open frequently, especially by chewing insects and larger animals. Two mechanisms seal broken sieve elements. The first is P-pro- tein (P for phloem), found as a fine network adjacent to the plasma membrane inner surface of uninjured sieve elements. When phloem is ruptured, the phloem sap initially surges toward the break; this rapid movement sweeps the P-protein into the cell center, where it becomes a tangled mass. When it is carried to a sieve area or sieve plate, the P-protein mass is too large to pass through and forms a P-protein plug (Fig. 2).
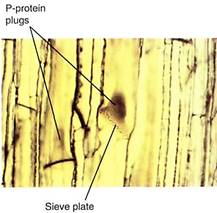
FIGURE 2: When this material of squash (Cucurbita) was being prepared for microscopy, it was cut open, causing the phloem sap to surge toward the cut, sweeping P-protein along and forming P-protein plugs, visible as dark brown masses. Sieve pores in the sieve plates are also visible (X 500).
Within uninjured phloem there is another polymer as well, callose. Apparently, it can stay in solution only if it is under pressure; without pressure it precipitates and forms a flocculent mass. When injury causes a pressure drop, the callose precipitates and is carried along with the P-protein to the nearest sieve areas; there the callose contributes to the plug and leaking is prevented.
In monocots with long-lived stems, such as palms and Joshua trees, sieve tube members live and function for many years, even hundreds of years. But in all other plants, individual sieve elements have a lifetime of only months or even weeks. They stop transporting and are replaced by new phloem cells from the provascular tissues or vascular cambium. Once they cease to function, callose deposits seal them permanently.
A further aspect of phloem transport is important to consider. As sugar is actively transported into phloem in sources, what happens to the water potential of the cells losing the sugar? Shouldn't it become less negative? No, it remains unchanged, because the sugars are being exported at the same rate they are being synthesized in leaves. Chlorenchyma cells absorb carbon dioxide molecules, but this does not cause the water potential to become more negative because the carbon dioxide is synthesized into sugar and exported. Millions of molecules of carbon dioxide may pass through a chlorenchyma cell without any long-term impact on its osmotic or water potentials. In sources such as tubers, the export of sugar has no impact on the storage cell water potential because as rapidly as it is exported, new sugar appears by the depolymerization of starch. The same is true at sinks: Storage cells do not accumulate sugar as sucrose but polymerize it into starch. Thousands of molecules may be absorbed, but because they are polymerized into one molecule, no change in water potential occurs. In growing cells of sinks such as meristems and buds, the imported sugar is polymerized to cellulose, hemicellulose, and other carbohydrates. It can also be metabolized into amino acids, fatty acids, and nucleotides; these are then polymerized into proteins, fats, and nucleic acids, so again little or no change in osmotic potential and water potential occurs. In all cells, part of the imported sugar is respired, but this converts it to carbon dioxide that is expelled, so again there is no osmotic effect.
Plants control the direction and rate of flow of phloem sap. While dormant in late winter and early spring, buds receive very little phloem sap, but once they become active, phloem transport increases greatly. Not all buds are equally affected; some grow rapidly whereas others, even though located quite close by, receive virtually nothing. While flowers are open, phloem transport is low, but after fertilization, when the ovary begins to develop into a fruit, transport increases.
The direction of transport can also change. Leaf primordia and young expanding leaves are sinks; imported sugar allows them to develop much more rapidly than they would if they had to be completely autotrophic . In addition, they also need large amounts of nitrogen, sulfur, potassium, and other minerals. Once leaves reach a critical size, they become self-supporting, able to photosynthesize rapidly enough to meet all their own needs (Fig. 3). Shortly afterward they become sources, exporting material. Phloem transport is reversed in the leaf and petiole; molecular pumps must now load the phloem, not unload it. Plasma membranes may be altered, or one set of sieve elements may cease to function and may be replaced by a whole new set of cells. The early primary phloem often lives for less than a few weeks.
Leaves near a stem tip export upward to the shoot apical meristem, while leaves farther back export toward the trunk and roots. As the shoot apex grows, leaves that had been near the apex are left behind and their transport shifts from upward to downward. If the apex produces flowers and then fruit, the direction of transport may shift again so that all leaves send sugars upward.

FIGURE 3:This bean contains two prominent sources, the cotyledons, which are supplying sugars, amino acids, and minerals to the rest of the seedling. The shoot tip with its meristem and leaf primordia are sinks, as are the roots. The first two leaves are expanded and are probably sources now, but they were sinks while they were developing.
 الاكثر قراءة في مواضيع عامة في علم النبات
الاكثر قراءة في مواضيع عامة في علم النبات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


