


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 1-3-2016
Date: 18-3-2016
Date: 6-3-2016
|
Presence/absence method BAM/FDA 2011 for Salmonella in foods
Food and Drug Administration (FDA) method, as described in 2011 Version of the Bacteriological Analytical Manual Online (Andrews and Hammack, 2011). It is applicable to all foods intended for human consumption.
1. Material required for analysis
• Enrichment Broth (vary as a function of the sample to be examined, consult Table.1)
• Sodium hydroxide (NaOH) 1N solution sterile
• Hydrochloric Acid (HCl) 1N Solution sterile
• Rappaport-Vassiliadis (RV) Medium
• Tetrathionate (TT) Broth
• Selenite Cystine (SC) Broth
• Hektoen Enteric (HE) Agar
• Bismuth Sulfite (BS) Agar
• Xylose Lysine Desoxycholate (XLD) Agar
• Triple Sugar Iron Agar (TSI)
• Lysine Iron Agar (LIA)
• Formalinized Physiological Saline Solution
• Salmonella polyvalent flagellar (H) antiserum
• Salmonella Spicer-Edwards flagellar (H) antisera
• Salmonella polyvalent somatic (O) antiserum
• Miniaturized identification kit API 20E (BioMeriéux Vitek) or Vitek GNI (BioMeriéux Vitek) or Enterotube II (Beckton Dicson) or Enterobacteriaceae II (Beckton Dicson) or Micro ID (Remel) or bio-chemical tests media bellow
• Urea Broth or Urea Broth Rapid
• Decarboxylase Broth Falkow 0.5% L-Lysine
• Phenol Red Carbohydrate Broth or Purple Broth
with 0.5% Dulcitol
• Phenol Red Carbohydrate Broth or Purple Broth
with 0.5% Lactose
• Phenol Red Carbohydrate Broth or Purple Broth
with 0.5% Sucrose
• Tryptone (Tryptophane) Broth
• Potassium Cyanide (KCN) Broth
• Malonate Broth
• Trypticase (Tryptic) Soy Broth (TSB) or Brain Heart
Infusion (BHI) Broth
• Simmons Citrate Agar
• Indole Kovacs Reagent
• Voges-Proskauer (VP) Test Reagents (5% α-naphthol
alcoholic solution, 40% potassium hydroxide
aqueous solution, creatine phosphate crystals)
• Methyl Red Solution
• Water bath (circulating) set to 42 ± 0.2°C
• Water bath (circulating) set to 43 ± 0.2°C
• Laboratory incubator set to 35 ± 2°C
• Laboratory incubator set to 37 ± 0.5°C
• Water bath set to 48–50°C
2. Procedure
A general flowchart for detection of Salmonella in foods using the presence/absence method BAM/FDA 2011 is shown in Figure 1.
a) Pre-enrichment: homogenize 25 g or 25 ml of sample with 225 ml of enrichment broth (use Table 1 and notes bellow to select the enrichment broth). Cap the flask securely and let stand 60 ± 5 min at room temperature. Mix well and determine the pH. Adjust the pH, if necessary, to 6.8 ± 0.2 with sterile 1N NaOH or 1N HCl (mix well before determining final pH). Incubate at 35 ± 2°C/24 ± 2 h with the flask caps slightly loosened.
Note a.1) For the analysis of frozen foods, it is not recommended to thaw the samples before pre-enrichment, not only to prevent injuries to Salmonella cells, but also to reduce multiplication of competitors. If it is not possible to withdraw the analytical unit with-out thawing, an appropriate amount of the sample should be thawed in a water bath under agitation, at a temperature lower than 45ºC and for no longer than 15 minutes. Alternatively, thaw in the refrigerator (2 to 5ºC) for 18 h.
Note a.2) For the analysis of powdered, low solubility foods (dry mixes for the preparation of soups, powdered eggs, etc.), it is recommended to add the pre-enrichment broth gradually and under constant agitation to avoid lumping. Add to the 25 g sample a small initial volume of Lactose Broth (15–20 ml) and use a glass stirrer or a magnetic stirrer to facilitate homogenization (previously sterilize the glass stirrer or the magnetic stirring bar). Always under agitation, add more 10 ml of the broth and repeat this procedure for a third time. Finally, add the remaining volume of broth and keep under agitation until obtaining a homogeneous suspension.

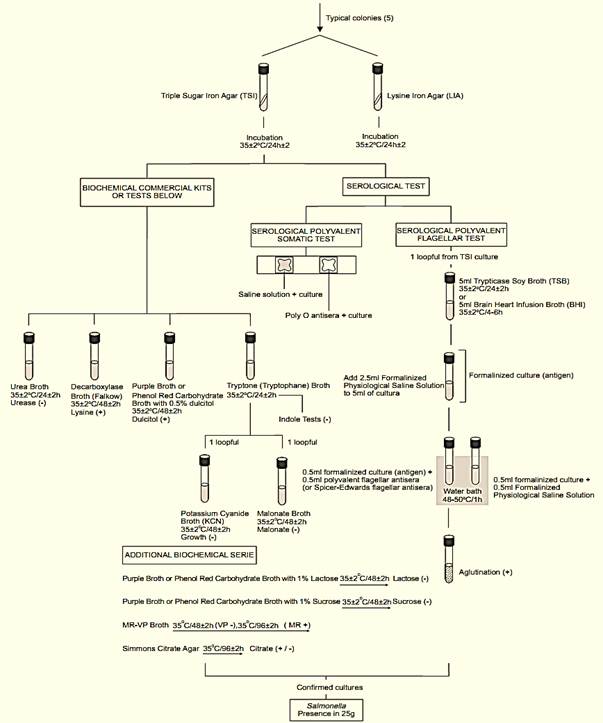
Figure 1 Scheme of analysis for detection of Salmonella in foods using the presence/absence method BAM/FDA 2011 (Andrews and Hammack, 2011).
Note a.3) For the analysis of milk powder, it is recommended to add the sample to the enrichment broth in small portions, spreading each portion in thin layers onto the surface of the liquid, without agitation, until it is completely absorbed. Let stand for 60 ± 5 min, without agitation. It is not necessary to adjust the pH before the incubation.
Note a.4) For the analysis of the internal content of fresh (“in natura”) eggs, it is recommended to wash the eggs under running water, using a brush to remove any material that may have adhered to the shells. Immerse and keep the eggs for 10s in a 3:1 alcoholic solution of iodine, remove and leave to dry in a laminar flow chamber. Break the shells aseptically, and transfer the internal content to a sterile plastic bag and, securing the bag with the hands on the outside, homogenize until the yolk is completely and evenly mixed with the egg white. Incubate at 20–24°C/96 ± 2 h and, after incubation, withdraw the 25 g-analytical unit. Boiled eggs with their shells intact should be disinfected and prepared in the same way.
Note a.5) For the analysis of food dyes and colorants there are two procedures: Dyes with pH 6.0 or above (10% aqueous suspension) may be examined in the normal way. For dyes with pH below 6.0 (10% aqueous suspension), the pre-enrichment step may be suppressed, adding 25 g of the sample directly to 225 ml of Tetrathionate Broth (TT) not supplemented with Brilliant Green. Mix well, let stand for 60 ± 5 min at room temperature, agitate and with-draw an aliquot of a known volume to determine the pH. If necessary, adjust to 6.8 ± 0.2 and add 2.25 ml of a 0.1% Brilliant Green solution. Agitate and incubate at 35 ± 2°C/24 ± 2 h with the screw cap slightly loose. This step corresponds to the selective enrichment step and is directly followed by differential selective plating.
Note a.6) For the analysis of gelatin, weigh 25 g of the sample and add 225 ml of Lactose Broth and 5 ml of a 5% aqueous papain solution (5 g of papain in 95 ml sterile distilled water). Homogenize in a stomacher or by agitation, cap the jar securely and incubate at 35 ± 2°C/60 ± 5 min. Agitate well, adjust the pH to 6.8 ± 0.2 and incubate at 35°C/24 ± 2 h, with the screw cap slightly loose.
Note a.7) For the analysis of guar gum, add 225 ml Lactose Broth and 2.25 ml of an 1% aqueous solution of cellulase (1 g of cellulase in 99 ml sterile distilled water, sterilized by filtration) in a sterile jar or flask. Place the broth in a magnetic agitator (previously sterilize the magnetic stirring bar) and add 25 g of the sample to the broth, under constant and vigorous agitation. Close the flask or jar tight, and leave to stand for 60 ± 5 min at room temperature. Next, incubate at 35 ± 2°C/24 ± 2 h with the screw cap slightly loose. Here, it is not necessary to adjust the pH value.
Note a.8) For the analysis of rabbit carcasses, transfer the carcass to a sterile plastic bag and weigh. Add Lactose Broth in the quantity required to obtain a 10−1 dilution. Agitate well, leave to stand for 60 ± 5 min at room temperature and adjust the pH to 6.8 ± 0.2, if necessary. Incubate at 35 ± 2°C/24 ± 2 h.
Note a.9) For the analysis of whole melons and tomatoes, immerse the fruit in Universal Pre-Enrichment Broth (UP), in the quantity required to keep the fruit afloat (approximately 1.5 times the weight of the melons and one time the weight of the tomatoes). Without agitating, leave to stand for 60 ± 5 min at room temperature and, without adjusting the pH, incubate at 35 ± 2°C/24 ± 2 h.
Note a.10) For the analysis of whole mangos, immerse the fruit in Buffered Peptone Water (BPW), in the amount necessary to keep the fruit afloat (approximately one time the weight of the fruit). Without agitation, leave to stand for 60 ± 5 min at room temperature, adjust the pH to 6.8 ± 0.2 (if necessary and) and incubate at 35 ± 2°C/24 ± 2 h.
Note a.11) For the analysis of environmental samples (swabs and sponges). Immediately after collecting the samples, immerse in DeyEngley Broth (DE), in the amount necessary to complete cover the swab or sponge. Transport in a Styrofoam box with gel ice. Store under refrigeration (4 ± 2°C) until analysis, which should be performed within 48 hours. Start the test by transferring the swab or sponge to a flask or jar containing 225 ml Lactose Broth, agitate and leave to stand for 60 ± 5 min at room temperature. Agitate well, adjust the pH to 6.8 ± 0.2 (if necessary) and incubate at 35 ± 2°C/24 ± 2 h.
Note a.12) For the analysis of mamey pulp, homogenize 25 g of the sample with 225 ml Universal Pre-Enrichment Broth. Leave to stand for 60 ± 5 min at room temperature. Next, incubate at 35 ± 2°C/24 ± 2 h with the screw cap tightly loose, without adjusting the pH. If there is any suspicion of contamination of the sample with Salmonella Typhi, prepare the Universal Pre-Enrichment Broth without ammonium ferric citrate. Treat these samples as products with a low bacterial load.
Note a.13) For the analysis of frog legs, place 15 pairs of legs into sterile plastic bag and add Lactose Broth in the quantity required to obtain a 10−1 dilution. Mix well and let stand 60 ± 5 min at room temperature. Mix well, determine the pH and adjust, if necessary, to 6.8 ± 0.2. Incubate 35 ± 2°C/24 ± 2 h.
b) Selective enrichment: After the incubation period inoculate 0.1 ml of the pre-enrichment broth into 10 ml tubes of Rappaport-Vassiliadis Medium (RV) and 1 ml into 10 ml tubes of Tetrathionate Broth (TT). Incubate the RV tubes at 42 ± 0.2°C/24 ± 2 h (circulating, thermostatically-controlled, water bath). Incubate the TT tubes at 35 ± 2°C/24 ± 2 h (foods with a low microbial load) or 43 ± 0.2°C/24 ± 2 h (circulating, thermo-statically controlled, water bath) (foods with a high microbial load).
Table 1 Guide for selecting pre-enrichment broths, dilution ratios and eventual variations in the pre-enrichment procedure for Salmonella analysis using the method BAM/FDA (Andrews and Hammack, 2011).


1. Add the enrichment broth gradually and under constant agitation, to prevent lumping. Add to the 25 g sample a small initial amount of broth (15–20 ml) and use a glass stirrer or a magnetic agitator to facilitate homogenization, always previously sterilizing the glass stirrer or magnetic stirring bar. Always under agitation, add more 10 ml of the broth and repeat this procedure for a third time. Finally, add the remaining volume of broth and keep under agitation until obtaining a homogeneous suspension.
2. Add the sample to the enrichment broth in small portions, spreading each portion in thin layers onto the surface of the liquid, without agitation, until it is completely absorbed.
3. Do not adjust the pH nor agitate after the 60 ± 5 min holding time.
4. After homogenizing the sample, leave to stand for 60 ± 5 min at room temperature, adjust the pH at 6.8 ± 0.2 and add 2.25 ml of a 0.1% Brilliant Green Solution. Incubate at 35 ± 2°C/24 ± 2 h and pass on directly to differential plating.
5. The 60 ± 5 min holding time should be completed at 30°C.
6. Decontaminate the shells in a 3:1 alcoholic solution of iodine.
7. Decontaminate the shell (peel) in a 3:1 alcoholic solution of iodine, if it is intact.
8. For this product the pooling before pre-enrichment should not be used.
9. Manually mix the content by vigorously swirling the flask 25 times clockwise and 25 times counterclockwise. Leafy green vegetables should be analyzed without cutting the leaves.
Note b.1) For guar gum and foods suspected to contain Salmonella Typhi, use Selenite Cystine Broth (SC) instead of RV (1 ml of the pre-enrichment broth into 10 ml of SC). Incubate SC and TT at 35 ± 2°C/24 ± 2 h.
Note b.2) There are many formulations of the Tetrathionate Broth commercially available. The formulation recommended by the BAM/FDA method is Mueller’s modified by Kauffmann, sold with the codes: Tetrathionate Broth Base Difco 210430, Merck 1.05285, Oxoid CM 671, Acumedia 7241.
c) Selective-differential plating: From the culture obtained in TT broth streak a loopful (10 μl) onto a plate of Bismuth Sulfite Agar (BS), a loopful (10 μl) onto a plate of Xylose Lysine Desoxycholate Agar (XLD) and a loopful (10 μl) onto a plate of Hektoen Enteric Agar (HE). Repeat the same procedure with the culture obtained in RV (or SC).
Incubate the plates (inverted) at 35 ± 2°C/24 ± 2 h and verify the presence of typical colonies. Reincubate the BS plates and repeat examination after 48 ± 2 h of incubation.
Note c.1) The BAM/FDA recommends prepare BS plates the day before using and store in dark at room temperature.
Typical Salmonella colony morphology:
HE: On HE the Salmonella colonies are blue or blue-green (lactose not fermented) and the center may be black (H2S produced) or not (H2S not produced). Some strains of Salmonella produce large amounts of H2S resulting in colonies with large black centers or almost completely black. A few Salmonella strains are lactose positive and produce atypical yellow colonies, with or without black centers.
XLD: On XLD the Salmonella colonies are pink (lactose not fermented) and the center may be black (H2S produced) or not (H2S not produced). Some strains of Salmonella produce large amounts of H2S resulting in colonies with large black centers or almost completely black. A few Salmonella strains are lactose positive and produce atypical yellow colonies, with or without black centers.
BS: On BS the Salmonella colonies are brown, gray, or black, with or without a metallic sheen, and show a brown halo which may become black with a prolonged incubation. Some strains produce atypical green colonies with a small dark halo or without halo.
d) Screening: From each selective agar plate select two (or more) typical colonies for confirmation. If no typical colonies are present on HE and XLD, select two atypical (yellow). If no typical colonies are present on BS after 24 h of incubation, reincubate the plates and select the colonies after 48 ± 2 h of incubation. If no typical colonies are present on BS after 48 h of incubation, then select two atypical (green).
From each selected colony inoculate a tube of Triple Sugar Iron Agar (TSI) by streaking the slant and stabbing the butt. With the same inoculum, without flaming the needle, inoculate a tube of Lysine Iron Agar (LIA) by stabbing the butt twice and then streaking the slant. Incubate the TSI and LIA tubes at 35 ± 2°C/24 ± 2 h (with the caps slightly loosened to maintain aerobic conditions). After the inoculation maintain the HE, BS and XLD plates under refrigeration (5–8°C).
Note d.1) Since the lysine decarboxylation reaction is strictly anaerobic, the LIA slants must have a deep butt (4 cm).
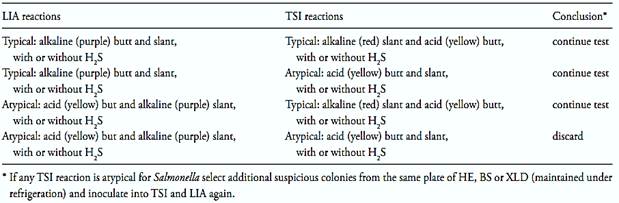
After the incubation period, examine the tubes for typical Salmonella reactions. In TSI: alkaline (red) slant and acid (yellow) butt, with or without production of H2S (blackening of agar). In LIA: alkaline (purple) reaction in butt of tube with or without production of H2S. Follow criteria from Table 2 to select cultures for biochemical and serological tests. For cultures giving atypical TSI reactions select additional suspicious colonies from the same plate of HE, BS or XLD maintained under refrigeration and inoculate TSI and LIA again.
e) Confirmation: Examine a minimum of six TSI cultures for each 25 g analytical unit or each 375 g composite. Use the cultures from TSI for bio-chemical and serological tests below. If necessary purify the cultures before confirmation by streaking on HE, XLD or MacConkey Agar, incubated at 35 ± 2°C/24 ± 2 h (on MacConkey agar the typical colonies appear transparent and colorless, some-times with dark center).
Table 2 Guide for selecting TSI and LIA cultures for confirmation tests according the method BAM/FDA 2011.
Confirm the cultures applying the tests described below. As alternative to conventional biochemical tests commercial kits may be used (API 20E, Enterotube II, Enterobacteriaceae II, MICRO-ID, or Vitek GNI), but they should not be used as a substitute for the serological tests.
e.1) Urease test (conventional): Inoculate the culture into a tube of Urea Broth and incubate the tubes at 35 ± 2°C/24 ± 2 h. Use an uninoculated tube of urea broth as control because the medium occasionally become purple-red (positive test) on standing. Optional urease test (rapid). Inoculate three loopful of the culture (heavy inoculum) into tubes of Rapid Urea Broth. Incubate the tubes at 37 ± 0.5°C/2 h (water bath) and examine for alkaline reaction (change in color to purple red). Salmonella strains are urease negative.
e.2) Serological polyvalent flagellar (H) test: Apply the polyvalent flagellar (H) test to each urease negative culture. The test may be per-formed at this point of the procedure or later, when performing the tests described in item e.3 below. To read the results on the same day, inoculate the cultures into tubes of Brain Heart Infusion Broth (BHI) and incubate at 35 ± 2°C until visible growth occurs (4 to 6 h). To read the results on the following day, inoculate the cultures into tubes of Trypticase Soy Broth (TSB) and incubate the tubes at 35 ± 2°C/24 ± 2 h. To perform the test, add 2.5 ml of Formalized Physiological Saline Solution to 5 ml of the BHI or TSB culture.
These formalized broth cultures are the anti-gen for the Salmonella polyvalent flagellar (H) antisera. Place 0.5 ml of the Salmonella poly-valent flagellar (H) antiserum in a tube and add 0.5 ml of the formalized broth culture. Prepare a control tube in parallel, with 0.5 ml of the Formalinized Physiological Saline Solution and 0.5 ml of the formalinized antigen. Incubate the tubes at 48–50°C/1 h in water bath. Observe at 15 min intervals and read the final results in 1 h. Positive result is indicated by agglutination in the test tube and no agglutination in the control tube. Negative result is indicated by no agglutination in the test tube and no agglutination in the control tube. Nonspecific result is indicated by agglutination in both tubes. The cultures giving nonspecific results should be tested with Spicer-Edwards antisera. The procedure is the same, using the Spicer-Edwards flagellar (H) antisera instead of the Salmonella polyvalent flagellar (H) antisera.
e.3) Testing urease negative cultures
e.3.1) Lysine decarboxylase test: This test is required only for cultures with doubtful LIA reaction. Inoculate the cultures into tubes of Decarboxylase Broth Falkow and incubate at 35 ± 2°C/48 ± 2 h. A positive test is indicated by a purple color throughout the medium (alkaline reaction). A negative test is indicated by yellow color throughout medium. Doubtful results (discolored medium neither purple nor yellow) may be confirmed by adding a few drops of 0.2% Bromcresol Purple Solution to the tubes. Salmonella strains are positive in this test.
e.3.2) Dulcitol fermentation test: Inoculate the cultures into tubes of Phenol Red Carbohydrate Broth or Purple Broth supplemented with 0.5% of dulcitol and containing an inverted Durham tube. Incubate the tubes at 35 ± 2°C/48 ± 2 h. Positive results are indicated by acid production (yellow color throughout the medium).
Negative tests are indicated by a red color throughout the medium (with phenol red as indicator) or a purple color throughout the medium (with bromcresol purple as indicator). Most Salmonella species give a positive test with gas formation in the Durhan tube.
e.3.3) Indole test: Inoculate the cultures into tubes of Tryptone (Tryptophane) Broth and incubate at 35 ± 2°C/24 ± 2 h. To perform the test transfer 5 ml of the 24 h culture to an empty test tube and add 0.2–0.3 ml of Indole Kovacs Regent. Observe for the development of a deep red color at the broth sur-face (positive test) or lack of red color (negative test). Intermediate orange and pink colors are considered as ±. Most Salmonella cultures give negative test. The remaining Tryptone (or Tryptophane) Broth should be used for performing the KCN and malonate growth tests.
e.3.4) Potassium Cyanide Broth (KCN) growth test: Caution, the KCN broth is poisonous. Prepare the medium using aseptic technique and stopper the tubes with corks impregnated with paraffin, which forms a seal between the rim of tubes and the cork. Inoculate the 24 h cultures obtained on Tryptone (Tryptophane) Broth into tubes of KCN Broth. Heat the rim of the tube before replacing the cork, to form a good seal. Incubate the tubes 35 ± 2°C/48 ± 2 h. Growth (turbidity) is indicative of a positive test and lack of growth is indicative of a negative test. Most Salmonella species do not grow in this medium.
e.3.5) Malonate test: Inoculate the 24 h cultures obtained on Tryptone (Tryptophane) Broth into tubes of Malonate Broth and incubate the tubes at 35 ± 2°C/48 ± 2 h. Use an uninoculated tube of Malonate Broth as control because the medium occasionally become blue (positive test) on standing. Positive test is indicated by a blue color throughout medium.
Negative test is indicated by a green (unchanged) color. Yellow color may occur (glucose fermentation) and is considered a negative test. Most Salmonella cultures give negative result (green or unchanged color) in this test.
Note e.3.5.1) At this point of the procedure, discard as not Salmonella any culture that shows either positive indole test and negative serological flagellar (H) test, or positive KCN test and negative lysine decarboxylase test.
e.4) Serological polyvalent somatic (O) test: Mark two sections (1 × 2 cm) on the inside of a Petri dish (use a wax pencil). Emulsify one loopful of the culture with 2 ml of 0.85% saline and add one drop of this emulsion to each marked section. To one section add one drop of Salmonella polyvalent somatic (O) antiserum and mix with the culture. To the other section add one drop of 0.85% saline and mix with the culture. Tilt the mixtures in a back-and-forth motion for one minute and observe for agglutination against a dark back-ground. A positive result is indicated by any degree of agglutination in the section containing the antiserum and no agglutination in the section containing the 0.85% saline control. Negative result is indicated by no agglutination in the section containing the antiserum and no agglutination in the section containing the 0.85% saline control. Nonspecific result is indicated by agglutination in both sections.
Note e.4.1) At this point of the procedure, classify as Salmonella those cultures which exhibit the following reactions: glucose fermentation in TSI positive (yellow butt), lysine decarboxylase positive in LIA (purple butt and slant) or in Lysine Decarboxylase Broth (Falkow), H2S positive in TSI and LIA, urease negative, dulcitol fermentation positive, KCN growth negative, malonate test negative, indole test negative, polyvalent flagellar test positive and polyvalent somatic test positive. For cultures exhibiting one or more atypical reactions, per-form further biochemical and serological tests below.
e.5) Additional biochemical tests: Perform additional biochemical tests for cultures exhibiting one or more atypical reactions in the items above, but purify the culture again before performing the additional tests.
e.5.1) Lactose and sucrose fermentation tests: Follow the same procedure described for the dulcitol fermentation test (e.3), using Phenol Red Carbohydrate Broth or Purple Broth supplemented with 1% lactose or 1% sucrose. The cultures lactose positive may be discarded as not Salmonella except when the original TSI and LIA tubes showed acid slant (TSI) and lysine positive reaction (LIA) or when the malonate test was positive. In this case the further tests below are required to determine if they are S. arizonae.
e.5.2.) Methyl red (MR) and Voges-Proskauer (VP) tests: Inoculate the cultures into tubes of MR-VP Broth and incubate at 35°C/48 ± 2 h. After 48 h transfer 1 ml of the culture to an empty tube for the VP test and reincubate the remainder of MR-VP broth an additional 48 h at 35°C for the MR test. To perform the VP test add (to 1 ml 48 h culture) the Voges Poskauer Test Reagents. First add 0.6 ml of the 5% α-naphthol alcoholic solution and shake. Then add 0.2 ml of the 40% KOH solution and shake. Finally add a few crystals of creatine and read the results after 4 h at room temperature. A positive test is indicated by the development of a pink-to-ruby red color through-out the medium. A negative test is indicated by the absence of the pink-to-red color. Most Salmonella are VP-negative. To perform the MR test add (to 5 ml of 96 h culture) 5–6 drops of Methyl Red Solution and read the results immediately. A positive test is indicated by a red color through-out the medium and yellow color is indicative of negative test. Most Salmonella are MR positive. Discard as not Salmonella, cultures that give positive KCN and VP tests and negative methyl red test.
e.5.3) Citrate test: Inoculate the cultures into tubes of Simmons Citrate Agar by streaking the slant and stabbing the butt. Incubate the tubes at 35°C/96 ± 2 h. A positive test is indicated by growth, usually accompanied by a color change from green to blue. Negative test is indicated by absence of growth (or very little growth) and no color change. Most Salmonella are citrate-positive.
f ) Interpretation of the results
f.1) Classify as Salmonella cultures that have reaction patterns below: Glucose (TSI) positiveLysine decarboxylase positive (LIA or Lysine Decarboxylase Broth Falkow)
H2S (TSI and LIA) positive
Urease negative
Dulcitol fermentation positive
Growth in KCN negative
Malonate test negative (majority of S. arizonae cultures are positive)
Indole test negative
Polyvalent flagellar test positive
Polyvalent somatic test positive
Lactose fermentation negative (majority of
S. arizonae cultures are positive)
Sucrose fermentation negative
Voges-Proskauer test negative
Methyl red test positive
Citrate test variable
f.2) Discard as not Salmonella cultures that give any of the reaction patterns below:
Urease test positive
Indole test positive and polyvalent flagellar
test negative
Indole test positive and Spicer Edwards
Flagellar test negative
Lysine decarboxylase negative and growth in
KCN positive
Lactose fermentation positive, except
Malonate positive cultures (test further to determine if they are S. arizonae) or cultures presenting acid slant in TSI and typical reactions in LIA.
Sucrose fermentation positive, except cultures presenting acid slant in TSI and typical reactions in LIA
Methyl red negative, Voges-Proskauer positive and Growth in KCN positive
f.3) For cultures identified using biochemical kits, interpret results according to the following guidelines:
f.3.1. Report as Salmonella those cultures classified as presumptive Salmonella with commercial biochemical kits when the culture demonstrates positive Salmonella somatic (O) test and positive Salmonella (H) test.
f.3.2. Discard cultures presumptively classified as not Salmonella with commercial biochemical kits when cultures conform to AOAC criteria for classifying cultures as not Salmonella (AOAC methods 967.25 to 967.28, 978.24, 989.12, 991.13, 994.04, and 995.20) (Horwitz, 2000).
f.3.3. For cultures that do not conform to f.3.1 or f.3.2, send to reference typing laboratory for definitive serotyping and identification.
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
Andrews, W.H. & Hammack, T.S. (2011) Salmonella. In: FDA (ed.) Bacteriological Analytical Manual, Chapter 5. [Online] Silver Spring, Food and Drug Administration.



|
|
|
|
اكتشاف تأثير صحي مزدوج لتلوث الهواء على البالغين في منتصف العمر
|
|
|
|
|
|
|
زهور برية شائعة لتر ميم الأعصاب التالفة
|
|
|
|
|
|
جمعيّة العميد وقسم الشؤون الفكريّة تدعوان الباحثين للمشاركة في الملتقى العلمي الوطني الأوّل
|
|
|
|
الأمين العام المساعد لجامعة الدول العربية السابق: جناح جمعية العميد في معرض تونس ثمين بإصداراته
|
|
|
|
المجمع العلمي يستأنف فعاليات محفل منابر النور في واسط
|
|
|
|
برعاية العتبة العباسيّة المقدّسة فرقة العبّاس (عليه السلام) تُقيم معرضًا يوثّق انتصاراتها في قرية البشير بمحافظة كركوك
|