


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 25-7-2019
Date: 10-3-2016
Date: 19-10-2020
|
Compounds classified as heterocyclic probably constitute the largest and most varied family of organic compounds. After all, every carbocyclic compound, regardless of structure and functionality, may in principle be converted into a collection of heterocyclic analogs by replacing one or more of the ring carbon atoms with a different element. Even if we restrict our consideration to oxygen, nitrogen and sulfur (the most common heterocyclic elements), the permutations and combinations of such a replacement are numerous.
Devising a systematic nomenclature system for heterocyclic compounds presented a formidable challenge, which has not been uniformly concluded. Many heterocycles, especially amines, were identified early on, and received trivial names which are still preferred. Some monocyclic compounds of this kind are shown in the following chart, with the common (trivial) name in bold and a systematic name based on the Hantzsch-Widman system given beneath it in blue. The rules for using this system will be given later. For most students, learning these common names will provide an adequate nomenclature background.
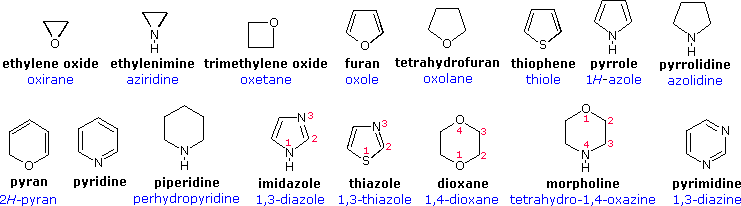
An easy to remember, but limited, nomenclature system makes use of an elemental prefix for the heteroatom followed by the appropriate carbocyclic name. A short list of some common prefixes is given in the following table, priority order increasing from right to left. Examples of this nomenclature are: ethylene oxide = oxacyclopropane, furan = oxacyclopenta-2,4-diene, pyridine = azabenzene, and morpholine = 1-oxa-4-azacyclohexane.
| Element | oxygen | sulfur | selenium | nitrogen | phosphorous | silicon | boron |
|---|---|---|---|---|---|---|---|
| Valence | II | II | II | III | III | IV | III |
| Prefix | Oxa | Thia | Selena | Aza | Phospha | Sila | Bora |
The Hantzsch-Widman system provides a more systematic method of naming heterocyclic compounds that is not dependent on prior carbocyclic names. It makes use of the same hetero atom prefix defined above (dropping the final "a"), followed by a suffix designating ring size and saturation. As outlined in the following table, each suffix consists of a ring size root (blue) and an ending intended to designate the degree of unsaturation in the ring. In this respect, it is important to recognize that the saturated suffix applies only to completely saturated ring systems, and the unsaturated suffix applies to rings incorporating the maximum number of non-cumulated double bonds. Systems having a lesser degree of unsaturation require an appropriate prefix, such as "dihydro"or "tetrahydro".
| Ring Size | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|
| Suffix Unsaturated Saturated |
irene irane |
ete etane |
ole olane |
ine inane |
epine epane |
ocine ocane |
onine onane |
ecine ecane |
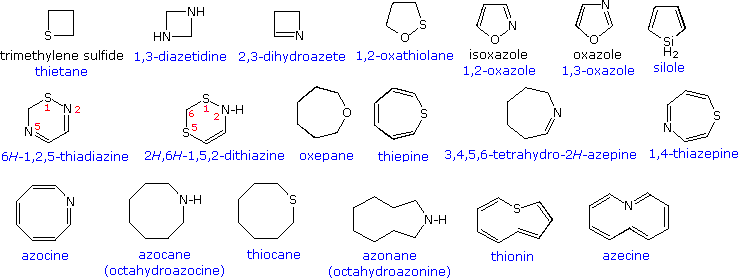
Despite the general systematic structure of the Hantzsch-Widman system, several exceptions and modifications have been incorporated to accommodate conflicts with prior usage. Some examples are:
• The terminal "e" in the suffix is optional though recommended.
• Saturated 3, 4 & 5-membered nitrogen heterocycles should use respectively the traditional "iridine", "etidine" & "olidine" suffix.
• Unsaturated nitrogen 3-membered heterocycles may use the traditional "irine" suffix.
• Consistent use of "etine" and "oline" as a suffix for 4 & 5-membered unsaturated heterocycles is prevented by their former use for similar sized nitrogen heterocycles.
• Established use of oxine, azine and silane for other compounds or functions prohibits their use for pyran, pyridine and silacyclohexane respectively.
Examples of these nomenclature rules are written in blue, both in the previous diagram and that shown below. Note that when a maximally unsaturated ring includes a saturated atom, its location may be designated by a "#H " prefix to avoid ambiguity, as in pyran and pyrrole above and several examples below. When numbering a ring with more than one heteroatom, the highest priority atom is #1 and continues in the direction that gives the next priority atom the lowest number.
All the previous examples have been monocyclic compounds. Polycyclic compounds incorporating one or more heterocyclic rings are well known. A few of these are shown in the following diagram. As before, common names are in black and systematic names in blue. The two quinolines illustrate another nuance of heterocyclic nomenclature. Thus, the location of a fused ring may be indicated by a lowercase letter which designates the edge of the heterocyclic ring involved in the fusion, as shown by the pyridine ring in the green shaded box.
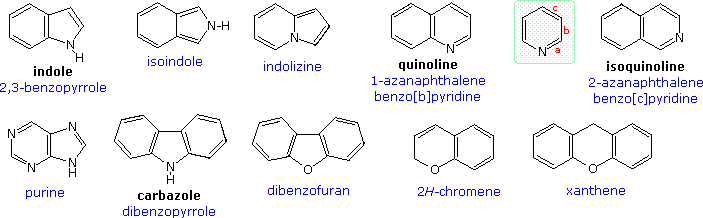
Heterocyclic rings are found in many naturally occurring compounds. Most notably, they compose the core structures of mono and polysaccharides, and the four DNA bases that establish the genetic code.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|