


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 3-9-2017
Date: 23-7-2017
Date: 14-8-2017
|
Dimethyl Carbonate (CO(OCH3)2)
Dimethyl carbonate (DMC) is a colorless liquid with a pleasant odor. It is soluble in most organic solvents but insoluble in water. The classical synthesis of DMC is the reaction of methanol with phosgene. Because phosgene is toxic, a non-phosgene-route may be preferred. The new route reacts methanol with urea over a tin catalyst. However, the yield is low.
Using electron donor solvents such as trimethylene glycol dimethyl ether and continually distilling off the product increases the yield.Dimethyl carbonate is used as a specialty solvent. It could be used as an oxygenate to replace MTBE. It has almost three times the oxygen content as MTBE. It has also a high octane rating. However, it must be evaluated in regard to economics and toxicity.

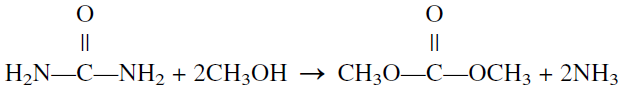



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|