


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 11-8-2017
Date: 22-12-2015
Date: 3-9-2017
|
SYNTHESIS GAS (STEAM REFORMING OF NATURAL GAS)
Synthesis gas may be produced from a variety of feedstocks. Natural gas is the preferred feedstock when it is available from gas fields (nonassociated gas) or from oil wells (associated gas). The first step in the production of synthesis gas is to treat natural gas to remove hydrogen sulfide. The purified gas is then mixed with steam and introduced to the first reactor (primary reformer). The reactor is constructed from vertical stainless steel tubes lined in a refractory furnace. The steam to natural gas ratio varies from 4–5 depending on natural gas composition (natural gas may contain ethane and heavier hydrocarbons) and the pressure used.
A promoted nickel type catalyst contained in the reactor tubes is used at temperature and pressure ranges of 700–800°C and 30–50 atmospheres, respectively. The reforming reaction is equilibrium limited. It is favored at high temperatures, low pressures, and a high steam to carbon ratio. These conditions minimize methane slip at the reformer outlet and yield an equilibrium mixture that is rich in hydrogen. The product gas from the primary reformer is a mixture of H2, CO, CO2, unreacted CH4, and steam. The main steam reforming reactions are:
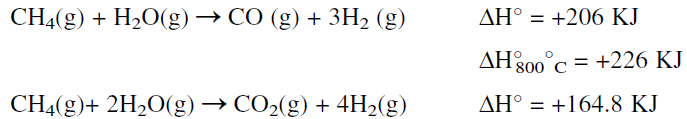
For the production of methanol, this mixture could be used directly with no further treatment except adjusting the H2/(CO + CO2) ratio to approximately 2:1. For producing hydrogen for ammonia synthesis, however, further treatment steps are needed. First, the required amount of nitrogen for ammonia must be obtained from atmospheric air. This is done by partially oxidizing unreacted methane in the exit gas mixture from the first reactor in another reactor (secondary reforming).
The main reaction occurring in the secondary reformer is the partial oxidation of methane with a limited amount of air. The product is a mixture of hydrogen, carbon dioxide, carbon monoxide, plus nitrogen, which does not react under these conditions. The reaction is represented as follows:
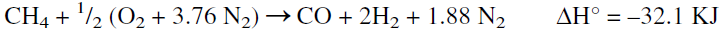
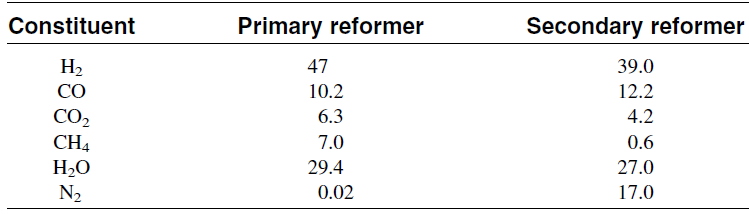
The reactor temperature can reach over 900°C in the secondary reformer due to the exothermic reaction heat. Typical analysis of the exit gas from the primary and the secondary reformers is shown in Table 1.1. The second step after secondary reforming is removing carbon monoxide, which poisons the catalyst used for ammonia synthesis. This is done in three further steps, shift conversion, carbon dioxide removal, and methanation of the remaining CO and CO2.
Table 1.1:Typical analysis of effluent from primary and secondary reformers



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|