


 تاريخ الرياضيات
تاريخ الرياضيات
 الرياضيات في الحضارات المختلفة
الرياضيات في الحضارات المختلفة 
 الرياضيات المتقطعة
الرياضيات المتقطعة
 الجبر
الجبر
 الهندسة
الهندسة 
 المعادلات التفاضلية و التكاملية
المعادلات التفاضلية و التكاملية 
 التحليل
التحليل
 علماء الرياضيات
علماء الرياضيات |
Read More
Date: 1-1-2016
Date: 11-1-2016
Date: 17-6-2019
|
In mathematics, there is no smallest number. It is always possible to find a number smaller than any number given. Zero is not the smallest number because any negative number is smaller than zero. The number line extends to infinity in both the positive and negative directions. However, when measuring things, it is often necessary to have a smallest number. If a car is stopped, it cannot go any slower. The temperature scale also has a lowest possible temperature, called “absolute zero.” This is somewhat confusing, because temperatures measured on either the Fahrenheit or Celsius temperature scales are often negative. In some countries, temperatures below zero are quite common in the winter. So, before talking about absolute zero, some temperature scales should be explored.
Temperature Scales
In the United States, temperatures are usually reported on the Fahrenheit temperature scale. On this scale, water freezes at 32° F and water boils at 212° F. Temperatures below zero (“negative temperatures”) are common, especially in the northern states. Thus 0° F is not the coldest possible temperature.
Scientific measurements of temperature in the United States and most other countries use the Celsius temperature scale. On this scale, water freezes at 0° C and water boils at 100° C. Nonetheless, 0° C is not the coldest possible temperature. In some parts of the United States, days or weeks may go by in winter when the temperature never rises above 0° C. Therefore, 0° C is also not the coldest possible temperature. However, there is a coldest possible temperature on both the Fahrenheit and Celsius scales, called absolute zero.
Determining Absolute Zero
Suppose an experiment is done to determine if there is a lowest possible temperature. Helium gas is put in a cylinder with a pressure gauge. Helium is chosen because the atoms are very small and the attractive forces between helium atoms are also very small. A gas whose atoms exert no forces on each other and whose atoms have no volume is called an “ideal gas.” Ideal gases do not exist, but helium behaves like an ideal gas if the temperature is relatively high (room temperature) and the pressure is low (atmospheric pressure).
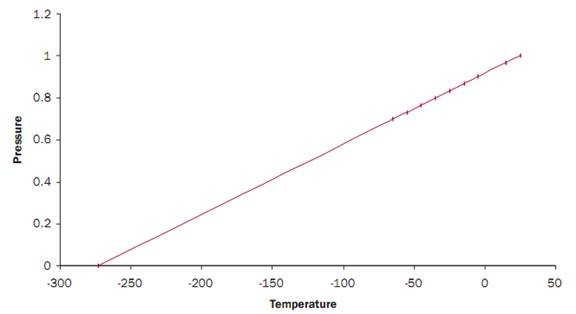
The cylinder and gas have an initial temperature of 25° C. The cylinder is placed in a refrigerator and the temperature in the refrigerator is lowered to 15° C. The pressure of the gas in the cylinder also goes down (becausethe gas molecules are going slower). If the temperature is lowered to 5° C, the pressure will go down even more. This is done several more times anda graph is drawn of the data (see figure).
Notice that all of the data points fall along a straight line. Now it can be asked, at what temperature will the gas have no pressure? Of course a real gas will turn to a liquid or solid at some low temperature, but the temperature at which an ideal gas would have no pressure can be extrapolated.
This temperature turns out to be approximately -273° C.
Although we picked helium gas for our thought experiment, the type of gas is not critical. At sufficiently high temperatures and low pressures, most gases behave like ideal gases. Consequently, at appropriately high temperatures and low pressures, all gases behave as if they would have no pressure at -273° C. This temperature is known as absolute zero.
Absolute Temperature Scales.
Scientists have defined an absolute temperature scale that starts at absolute zero and uses a unit the same size as the Celsius degree. This absolute temperature scale is called the Kelvin scale.
This temperature scale does not use a degree symbol. The unit of temperature is the kelvin. On this scale, water freezes at 273 K (read as 273 kelvin) and boils at 373 K. There is also an absolute temperature scale, called the “Rankine scale,” that uses a unit the same size as the Fahrenheit degree.
However, it is rarely used any more except in some engineering applications in the United States.
Achieving Absolute Zero.
Is it possible to build a refrigerator that will cool an object to absolute zero? Surprisingly, the answer is no. This is not just a matter of building a better refrigerator. There are theoretical reasons why a temperature of absolute zero is impossible to achieve. The impossibility of achieving absolute zero is usually called the third law of thermodynamics. But although it prohibits reaching absolute zero, it does not prevent obtaining temperatures as close to absolute zero as we wish to get.
Low Temperatures in Nature
What are the lowest temperatures that occur in nature? The lowest natural temperature ever recorded on Earth was 89°C (recorded in Vostok, Antarctica on July 21, 1983). Other objects in the solar system can have much lower surface temperatures. Triton, a satellite of Neptune, was observed by Voyager 2 to have a surface temperature of 37 K, making it the coldest known locale in the solar system. It is so cold that nitrogen freezes on the surface, making what looks like a pinkish frost. Triton has nitrogen geysers form when liquid nitrogen below the surface is vaporized by some internal heat source. The surface has lakes of frozen water. Pluto is only slightly warmer than Triton, with a temperature around 40 K to 60 K.
It might be thought that empty space is very cold. However, most space is not really empty. The space between the planets is not as cold as the surface of some of the planets. The few atoms and molecules in interplanetary space have a high kinetic energy (because of planetary magnetic fields and the solar wind), so they have a relatively high temperature. However, this hot gas will not warm an object in space, because there are so few atoms in the vacuum of interplanetary space.
The spaces between stars may also have quite high temperatures for the same reason as interplanetary space. Interstellar space can be filled with hot glowing gas, heated by nearby stars or strong stellar magnetic fields.
The interstellar gas can sometimes have a temperature of millions of kelvins.
However, the gas atoms are very far apart—farther apart than even in the best laboratory vacuum possible.
Intergalactic space is very cold. The radiation left over from the Big Bang at the beginning of the universe has a temperature of about 2.73 K.
However, on Earth we have been able to produce much lower temperatures in laboratories.
Artificial Low Temperatures
Although a refrigerator cannot be built that will reach absolute zero, for nearly 100 years we have known how to build a refrigerator that will produce a temperature lower than the lowest naturally occurring temperature.
Helium was first liquefied in 1908 by compressing and cooling the gas. Helium boils at 4.2 K. By rapidly pumping away the helium vapor, it is possible to lower the temperature to about 1.2 K. This happens because the helium atoms with the most energy are the ones that escape as vapor. As a result, pumping removes the higher energy atoms and leaves behind the atoms with lower kinetic energy.
The most common isotope of helium is helium-4. It has two neutrons and two protons in its nucleus. Helium-3 only has one neutron in its nucleus. By liquefying and pumping on a container of helium-3 it is possible to produce a temperature of around 0.3 K. This temperature is incredibly cold, but even lower temperatures have been recorded.
A mixture of helium-3 and helium-4 can produce even lower temperatures. At temperatures below 1.0 K, the mixture will separate into pure helium-3 and a saturated solution of helium-3 dissolved in helium-4. If the helium-3 atoms are pumped away from the mixture, more helium-3 atoms will dissolve from the pure helium-3 layer into the mixture. As might be expected, the helium-3 atoms with the most energy are the ones that will move into the mixture, leaving behind lower energy helium-3 atoms. This technique has produced temperatures as low as 0.002 K.
It is possible to produce even lower temperatures by using magnetic traps and other devices. At these extremely low temperatures, it becomes increasing difficult to state a precise definition of temperature. The nucleus of an atom, the conduction electrons of the atom, and the atom itself might all have different temperatures. The lowest temperature produced for liquid helium is 90 microkelvins.
The attempt to produce ever lower temperatures is not just competition for the sake of competition. The technology developed to produce and to measure low temperatures has broad applications in other fields. Also,the behavior of materials at extremely low temperatures tests the limits of our theoretical understanding of matter itself. For example, in 1996 Eric Cornell and a research team used magnetic trapping to produce a new state of matter, called a “Bose-Einstein condensate.” The existence of this state of matter confirmed our understanding of the quantum mechanical properties of matter.
______________________________________________________________________________________________
Reference
Arons, Arnold B. Development of Concepts of Physics. Reading, MA: Addison-Wesley Publishing Company, 1965.
Eisberg, Robert M., and Lerner, L. S. (1981). Physics: Foundations and Applications (Vol.II). New York: McGraw-Hill Book Company.
Epstein, Lewis Carroll. Thinking Physics. San Francisco: Insight Press, 1990.
Hewitt, Paul G. Conceptual Physics 2nd edition. Menlo Park CA: Addison-Wesley Publishing Company, 1992.
|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|