


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 27-11-2020
Date: 24-3-2021
Date: 4-5-2016
|
Coiled-Coils
A coiled-coil is a generic name for any helical structure that has an axis that is itself helical. A general feature of all such coiled-coil conformations is that the handedness of coiling alternates at successive levels of structure, in line with the well-established practice employed by ropemakers over the centuries. This maximizes the interactions between strands and minimizes relative slippage. There are two particularly well-known classes of coiled-coil structures in biological structures: (i( the a-fibrous proteins and (ii) the collagens. The structure of each will be described.
a-Fibrous proteins display a characteristic heptad quasi-repeat of the form (a–b–c–d–e–f–g)n, where about 75% of the a and d are positions are occupied by nonpolar residues such as leucine, isoleucine, and valine (1, 2). These sequences adopt a right-handed a-helical conformation with about 3.6 residues per turn. Because the apolar residues in the heptad repeat are 3.5 residues apart, on average, it follows that they will form an apolar stripe on the surface of the a-helix, and this will wind around the helix in a left-handed manner (3). In an aqueous environment, apolar residues tend to pack as closely together as possible to shield each other from the water and, in doing so, provide the hydrophobic driving force for assembly. This can be facilitated in this case by two or more a-helices coming together, optimizing the packing of the apolar residues along their interface (so-called knobs-into-holes packing) and winding around one another to generate a left-handed coiled-coil structure ) Fig. 1). Favorable electrostatic interactions can also be made between the chains, which help to specify both the relative chain direction and the axial displacement between the chains (1). The interchain ionic interactions occur predominantly between oppositely charged residues in positions e and g of different chains. Calculations and experimental observations on two-stranded a-helical coiled-coils have shown that the strands are parallel (rather than antiparallel) and in axial alignment.
The types of apolar residues in positions a and d are important in specifying the number of chains in the coiled-coil molecule. Two-stranded coiled-coils occur in muscle myosin, intermediate filaments, plectin, streptococcal M proteins, centrophilin, kinesin, b-giardin, and a host of other proteins. Three-stranded structures are found in the laminins, fibrinogen, the spectrin superfamily of proteins, bacteriophage leg proteins such as gp17, cartilage matrix protein, mannose-binding protein, macrophage scavenger receptor protein, and many others. Four-stranded ropes are found in the silks of the bees, wasps, and ants (Hymenoptera aculeata), as well as in globular proteins (such as the four-helix motif). Five-stranded coiled-coils have recently been described in HIV capsid protein and in collagen oligomeric matrix protein (COMP) (Fig. 2). As Cohen and Parry (3) have pointed out, it is not the bending of the axis of the a-helix in the supercoiled conformation that is crucial but rather the way in which it permits systematic apolar side-chain interactions to be made. It must also be pointed out that many sequences show discontinuities in their heptad substructures. A recent study by Brown et al. (4) has shown that all such (short) discontinuities can be classified as either “stutters” or “stammers”; these correspond to deletions of three and four residues, respectively, from an otherwise continuous repeat. Physically, a stutter results in a region in which the coiled-coil undergoes a degree of local underwinding to generate a longer supercoil pitch length, whereas the stammer causes a degree of local overwinding, thus giving rise to a shorter supercoil pitch length. The latter is likely to be stereochemically more difficult to achieve. The former has been observed experimentally. A coiled-coil is not confined to fibrous proteins with long rod-like domains as seen in myosin, intermediate filaments, and desmoplakin (for example), but occurs commonly in globular proteins in the form of a-helical bundles. These are generally short in length (say 3 to 10 heptads) and contain anything from two or three a-helices to sizable bundles containing six or even more. The same underlying heptad repeat is present, but it becomes less easy to recognize as the helix length decreases.
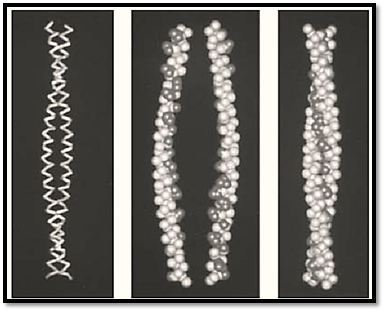
Figure 1. The structures of two-stranded coiled-coils. (Left) The backbone model of the two-stranded coiled-coil from a portion of the N-terminal end of tropomyosin. Two right-handed a-helices coil around each other in a left-handed manner. (Center) A space-filling model of the same structure with the strands shown separated. The apolar residues are shown in black. (Right) The two strands brought together. The apolar residues are interlocked in a systematic way along the axis of the coiled-coil and are shielded from water as a result.
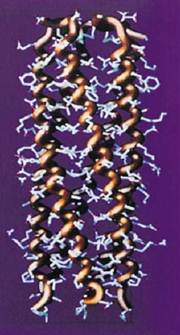
Figure 2. The five-stranded coiled-coil oligomerization domain of cartilage oligomeric matrix protein (COMP). Each of the a-helices forms about one-third of a complete turn over the length of the structure. The N-terminal end is at the bottom of the page.
The a-chains in collagen contain a triplet repeat of the form (Gly X Y)n, where X and Y can be almost any amino acid residue but are commonly proline and hydroxyproline, respectively. The chain folds up into a left-handed helical structure that is very similar to that seen in polyglycine II and polyproline II. Three a-chains then aggregate in parallel with a one-residue relative axial displacement between chains to generate a right-handed, triple-helical structure in which the glycine residues lie along the axis of the molecule. This class of conformation was originally formulated by Ramachandran and Kartha (5) and Rich and Crick (6) and has subsequently been refined by Fraser et al. (7, 8). The structure has 10 repeating units in three turns, a unit rise of 0.2894 nm, and a pitch length of 0.9647 nm. The individual a-chains have a pitch length of 8.68 nm. The conformation is stabilized by a single hydrogen bond per triplet between the peptide NH group of a glycyl residue and the peptide carbonyl group of an X residue in another chain. Furthermore, water molecules bridge the glycyl NH to the C=O of a prolyl residue in another chain and the OH group of a hydroxyprolyl residue in the same chain. The diameter of a collagen molecule is about 1.4 nm. Its length varies from one type of collagen to another, but for a Type I collagen molecule its length is close to 300 nm. The molecule thus has a high axial ratio in excess of 200.
References
1. J. F. Conway and D. A. D. Parry (1990) Structural features in the heptad substructure and longer range repeats of two-stranded -fibrous proteins. Int. J. Biol. Macromol. 12, 328–334.
2. J. F. Conway and D. A. D. Parry, Three-stranded -fibrous proteins: the heptad repeat and its implications for structure. Int. J. Biol. Macromol. 13, 14–16.
3. C. Cohen and D. A. D. Parry (1986) -Helical coiled-coils—a widespread motif in proteins. Trends Biochem. Sci. 11, 245–248.
4. J. H. Brown, C. Cohen, and D. A. D. Parry (1996) Heptad breaks in -helical coiled coils: stutters and stammers. Proteins Struct. Funct. Genet. 26, 134–145.
5. G. N. Ramachandran and G. Kartha (1955) Structure of collagen. Nature (London) 176, 593–595.
6. A. Rich and F. H. C. Crick (1961) The molecular structure of collagen. J. Mol. Biol. 3, 483–506.
7. R. D. B. Fraser, T. P. MacRae, and E. Suzuki (1979) Chain conformation in the collagen molecule. J. Mol. Biol. 129, 463–481.
8. R. D. B. Fraser, T. P. MacRae, A. Miller, and E. Suzuki, (1983) Molecular conformation and packing in collagen fibrils. J. Mol. Biol. 167, 497–521.
9. C. Cohen and D. A. D. Parry (1990) -Helical coiled-coils and bundles: how to design an -helical bundle. Proteins Struct. Funct. Genet. 7, 1–15.



|
|
|
|
إجراء أول اختبار لدواء "ثوري" يتصدى لعدة أنواع من السرطان
|
|
|
|
|
|
|
دراسة تكشف "سببا غريبا" يعيق نمو الطيور
|
|
|
|
|
|
اللجنة التحضيرية للمؤتمر الحسيني الثاني عشر في جامعة بغداد تعلن مجموعة من التوصيات
|
|
|
|
السيد الصافي يزور قسم التربية والتعليم ويؤكد على دعم العملية التربوية للارتقاء بها
|
|
|
|
لمنتسبي العتبة العباسية قسم التطوير ينظم ورشة عن مهارات الاتصال والتواصل الفعال
|
|
|
|
في جامعة بغداد.. المؤتمر الحسيني الثاني عشر يشهد جلسات بحثية وحوارية
|