
Enzymes
 المؤلف:
S. J. Benkovic
المؤلف:
S. J. Benkovic
 المصدر:
Ann. Rev. Biochem.
المصدر:
Ann. Rev. Biochem.
 الجزء والصفحة:
الجزء والصفحة:
 4-5-2016
4-5-2016
 3406
3406
Enzymes
Enzymes are proteins that act as biological catalysts. They are produced by living cells, but are independent of those cells for their catalytic activity. Like all catalysts, they speed up the rates of chemical reactions, but their special importance lies in the fact that they can do this at neutral pH and relatively low temperatures, and do so specifically, essentially for only one chemical reaction. The rate increase, which is due to the lowering of the activation energy barrier by binding and stabilizing the transition state, can be as much as 1012-fold (1). Under these circumstances, the uncatalyzed reaction would be barely detectable. As catalysts and under in vitro conditions, enzymes function at concentrations very much lower than those of the substrates on which they act. However, in the cell, the enzyme level often exceeds the substrate concentration. Enzymes increase the rate of a reaction in both the forward and reverse directions and, therefore, have no effect on the equilibrium constant Keq of the reaction. In theory, enzymes as catalysts remain unchanged at the end of a reaction, but as enzymes are proteins, they are subject to denaturation, which can lead to loss of activity over the course of a reaction. Enzymes may show either absolute or broad specificity. For example, catalase acts only on hydrogen peroxide, whereas alcohol dehydrogenase oxidizes a number of aliphatic and aromatic alcohols.
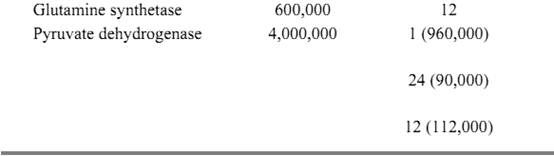
Enzymes have the same general structure as all proteins. The basic structure consists of a polypeptide chain that is made up of 20 amino acid residues joined in a linear sequence by peptide bonds. The folding of the polypeptide chain gives rise to the final enzyme structure, which contains an active site at which the substrates bind to undergo reaction. It is this folding to form the active site that is responsible for the catalytic power and specificity of enzymes. The active form of an enzyme may consist of single or multiple subunits, with molecular weights ranging from about 14,000 to 4,000,000 (Table 1).
Table 1. Molecular Weights and Subunit Composition of Selected Enzymes

Some enzymes bring about energy transductions that include the conversion of light energy to chemical bond energy in photosynthesis, as well as the conversion of chemical bond energy to mechanical energy in muscle contraction and to pumping energy for the membrane transport of ions against an unfavorable gradient.
The activities of enzymes are usually subject to control, not only through changes to intracellular substrate levels, but also by activation, inhibition, and covalent modification. The activity of enzymes is influenced by pH because of the presence of ionizing amino acid residues at the active site. Some enzymes require prosthetic groups or metal ions, whereas others must undergo irreversible or reversible activation. Reversible activation is often achieved by means of phosphorylation or adenylylation. Glycogen phosphorylase is activated by the transfer of a phosphoryl group from ATP to a single serine residue and inactivated by a phosphatase that removes the phosphoryl group. By contrast, glycogen synthase is inactivated by the phosphorylation of a serine residue and activated through phosphatase action. The protein kinases responsible for the phosphorylation of phosphorylase and glycogen synthase are activated by 3′, 5′-cyclic AMP. The interaction of this nucleotide with the regulatory subunits on the inactive kinases causes release of the active forms of the enzymes. The reversible activation of glutamine synthetase involves the transfer of the AMP moiety of ATP to and from the hydroxyl group of a tyrosine residue. Both reactions are catalyzed by the same enzyme, adenyl transferase . Enzymes can be inactivated by small molecules that covalently interact with amino acid residues at the active site or inhibited by substrate analogues, which can behave as drugs or pesticides.
A group of enzymes that are subject to special control are the allosteric enzymes. The activities of these enzymes are not only more sensitive to changes in intracellular substrate concentrations, but they are also activated and/or inhibited reversibly by modifiers that are small molecules which combine at either a specific binding site on a catalytic subunit or on a special regulatory subunit. Modifiers are frequently the end-product of a metabolic pathway and have structures that differ from those of the substrates for the allosteric enzymes. The control of enzyme activity can also be achieved through their interaction with low-molecular-weight proteins, such as calmodulin.
It has been demonstrated recently that it is possible to produce monoclonal antibodies which show catalytic activity (2). The generation of catalytic antibodies, or abzymes, has occurred through the use of an antigen that resembles the transition state or an intermediate state of the reaction. The idea was to produce an antibody that would bind tightly to, and stabilize, the transition state complex so as to enhance the reaction rate. The rate enhancement and small number of antibodies produced with a particular antigen suggest that the analogues are not good mimics of the actual transition state, or that good catalytic activity requires more than simply the stabilization of a transition state complex. Catalytic activity is not restricted to proteins, for RNA molecules have been shown to function as enzymes. These relatively small molecules, which are generally found in viruses, have catalytic features in common with those of enzymes (3).
Enzyme Nomenclature
In 1956, the International Union of Biochemistry established an International Commission on Enzymes. There were several reasons why. The number of known enzymes was increasing rapidly and their naming by individual researchers was not satisfactory. Further, the names did not always convey the nature of the reaction being catalyzed and similar names were given to enzymes of different types. The first report on enzyme nomenclature was produced in 1961 and contained references to 712 enzymes. The sixth report was published in 1984 by Academic Press and contains classifications for 2,477 enzymes. The publication lists classes of enzymes as well as information about the reference number, recommended name, reaction catalyzed and other names for particular enzymes. Enzymes in this volume are categorized as Dehydrogenases, Hydrogenases, Hydrolases, Isomerases, Synthetases/Ligases, Synthases/Lyases, Oxidoreductases, Phosphotransferases, and Transferases .
References
1. L. Frick, J. P. MacNeela, and R. Wolfenden (1987) Bioorg. Chem. 15, 100–108.
2. S. J. Benkovic (1992) Ann. Rev. Biochem. (1992) 61, 29–54.
3. W. G. Scott and A. Klug (1996) Trends Biochem. Sci. 21, 220–224.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


