


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 8-12-2020
Date: 6-11-2015
Date: 9-12-2015
|
Immune Defenses against Infection and Tumor Immunity
Protection against infections can be mediated by either; non-specific defense mechanisms (interferons, NK cells), or specific immunity in the form of antibodies and T cells which release cytokines and mediate contact- and perforin-dependent cell lysis. Control of cytopathic viruses requires soluble factors (antibodies, cytokines), whilst control of noncytopathic viruses and tumors is more likely to be mediated via perforins and cytolysis. However, cytotoxic immune responses can also cause disease, especially during noncytopathic infections. Development of an evolutionary balance between infectious agents and immune responses is an ongoing process, as reflected by the numerous mechanisms employed by pathogens and tumors to evade immune-mediated defenses.
All immune defense mechanisms are important in the battle against infections. Natural humoral mechanisms (antibodies, complement, and cytokines) and cellular mechanisms (phagocytes, natural killer cells, T cells) are deployed by the immune system in different relative amounts, during different phases of infection, and in varying combinations. Gross simplifications are not very helpful in the immunological field, but a small number of tenable rules can be defined based on certain model infections. Such models are mainly based on experiments carried out in mice, or on clinical experience with immunodeficient patients.
General Rules Applying to Infection Defenses
-Non-specific defenses are very important (e.g., Toll-like receptors, IFNa/b), and 'natural immunity' (meaning not intentionally or specifically induced) represented by natural antibodies, direct complement activation, NK cell and phagocytes, plays a significant role in all infections. However, much remains to be learned about their roles.
-Antibodies represent potent effector molecules against acute bacterial infections, bacterial toxins, viral re-infections, and in many cases against acute cytopathic primary viral infections (e.g., rabies and influenza). Antibodies are also likely to make a major contribution to the host-parasite balance occurring during chronic parasitic infections. IgA is the most important defense mechanism at mucosal surfaces.
-Perforin-dependent cytotoxicity in CD8+ Tcells is important for defense against noncytopathic viruses, for the release of chronic intracellular bacteria, and for protection against intracellular stages of certain parasites.
-Nonlytic T-cell responses provide protection in the form of cytokines (very important cytokines include IFNy and TNFa), which promote the enhanced digestion and destruction of intracellular bacteria and parasites (e.g., listeria, leishmania, etc.), and in some situations enhance immunity against complex viruses (e.g., the smallpox virus). Infectious agents apparently induce cytokines within a matter of hours (for instance IFNy, IL-12, and IL-4), and this early cytokine production in turn functions to define the ensuing T cell response as type 1 or type 2.
-IgE-mediated defense is important, along with IgA, in enhancing the elimination of gastrointestinal, pulmonary, and dermal parasites. Although details of the process are still sketchy, IgE-dependent basophil and eosinophil defense mechanisms have been described for model schistosomal infections.
-Avoidance strategies. Infectious agents have developed a variety of strategies by which they can sometimes succeed in circumventing or escaping immune responses, often by inhibiting cytokine action.
Antibacterial Immune Effector Mechanisms
Extracellular bacteria. Capsules with carbohydrate elements render bacteria more resistant to efficient phagocytosis and digestion (mainly by granulocytes)—how- ever, highly repetitive carbohydrate surface antigens induce efficient B cells responses which do not require T help and which are supported in part by lipopoly- saccharides (LPS). Pure carbohydrates do not induce T help! Short-lived IgM responses can control bacteria in the blood effectively, but are usually insufficient in the control of toxins. In such cases, immunoglobulins of the IgG class are more efficient, as a result of their longer half-life and greater facility for diffusing into tissues.
Intracellular bacteria are controlled by T cells (mainly via T cell secreted IFNy and TNFa which activate macrophages), or in some cases by the release of intracellular bacteria through CD8+ T cell mediated cellular destruction.
Avoidance Mechanisms of Pathogens (with examples)
Influence on the complement system. Some pathogens prevent complement factors from binding to their surfaces:
-Prevention of C4b binding; herpes virus, smallpox virus
Compartmentalization in non-lymphoid organs. Viruses can avoid confrontation with the immune defenses by restricting their location to peripheral cells and organs located outside of lymphoid tissues:
-Papilloma viruses; infect keratinocytes.
-Rabies virus; infects neurons.
Modulation and down-regulation of surface antigens. Infection agents can avoid immune defenses by mutating or reducing their expression of T- or B-cell epitopes.
-Influenza viruses; antigenic shift caused by rearrangement of genetic elements or drift resulting from mutation of hemagglutinin (at the population level).
Gonococci; recombination of pili genes.-
-Schistosoma; mutation of envelope proteins or masking by adoption of host MHC antigens.
Interference with phagocytosis and digestion. Mycobacterium tuberculosis uses CR1, CR2, or fibronectin as a receptor for cell entry; it does not induce efficient oxidative mechanisms in macrophages.
-Components of bacterial cell walls can impede phagosome-lysosome fusion and are resistant to digestion.
-Heat shock proteins (hsp60 and hsp70) or superoxidedismutase aid resistance.
Influence on lymphocytes and immunosuppression
-Direct destruction of lymphocytes, or negative regulation of their function (HIV).?
-Induction of immunopathological T-cell responses (in some cases these can be immunosuppressive, e.g. HIV).
-Induction of immunosuppressive autoantibodies.
Influence on selection, induction, and deletion of T cells
-Negative selection of T cells; if viral antigens are present in the thymus responsive T cells will be deleted
-Exhaustive activation, and subsequent deletion, of peripheral T cells; in some overwhelming peripheral virus infections all of the responding T cells are deleted (HBV, HCV).
Interference with cytokines, cytokine and chemotaxin receptors (R), etc. Many viruses produce substances that block or inhibit receptors for the humoral components of the immune defense system, for instance:
-IL-1 βR, TNFαR, IFNγR; herpesvirus, smallpox virus
. -Chemotaxin receptor; cytomegalovirus
-IL-10R; the Epstein-Barr virus produces B-cell receptor factor I, which binds to the IL-10R thus preventing activation of TH2 cells
-Viral-induced inhibition of interleukin production
Impairment of MHC antigen expression. Down-regulation of MHC class I and/or class II expression:
-Adenovirus; E19 protein reduces expression of MHC class I on infected cells.
-Murine cytomegalovirus; prevents transport of MHC class I to the Golgi apparatus.
Immune Protection and Immunopathology
Whether the consequences of an immune response are protective or harmful depends on the balance between infectious spread and the strength of the ensuing immune response. As for most biological systems, the immune defense system is optimized to succeed in 50-90% of cases, not for 100% of cases. For example, immune destruction of virus-infested host cells during the eclipse phase of a virus infection represents a potent means of preventing virus replication. From this point of view, lytic CD8+ T-cell responses make good sense as the host will die if proliferation of a cytopathic virus is not halted early on. If a noncytopathic virus is not brought under immediate control, the primary illness is not severe—however, the delayed cytotoxic response may then lead to the destruction of very large numbers of infected host cells and thus exacerbate disease (Tables 1 and 2). Since an infection with noncytopathic viruses is not in itself life-threatening to the
Table 1Balance between Infection and Host Immunity: Effect on the Disease
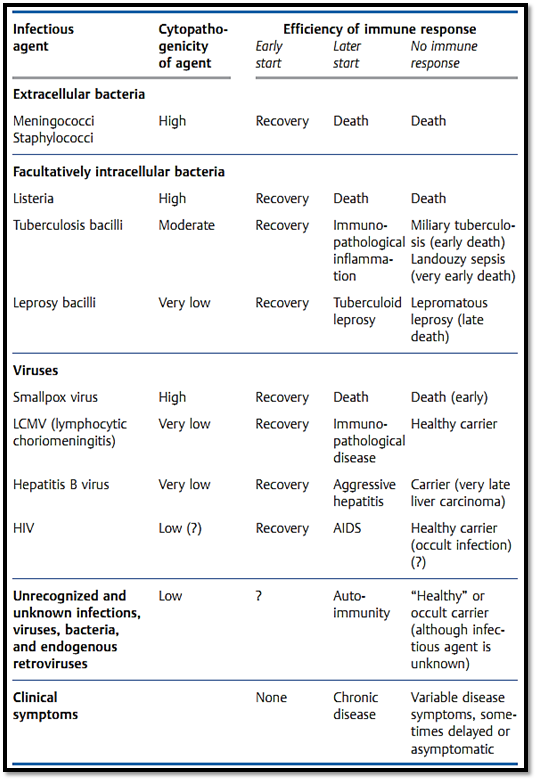
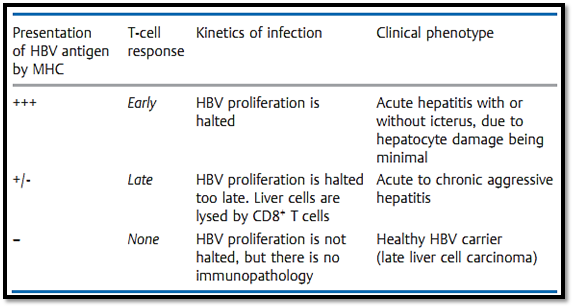
Table 2 Hepatitis B Virus (HBV) Infection. Inter-relations between Efficient Antigen Presentation by MHC Molecules, T-Cell Responses, Course of Infection, and Clinical Picture. Decreased Immunocompetence or Enhanced HBV Proliferation Shifts the Balance Towards an Unfavorable Outcome; Vaccination Shifts the Balance Towards a Favorable Outcome
host, it is paradoxically the immune response that is responsible for pathology and illness due to its ability to destroy infected host tissue.
Hepatitis B viral infections in humans (Table 2), and LCMV infections (lymphocytic choriomeningitis) in mice, are amongst the most thoroughly studied examples of this potentially negative consequence of protective immune responses. A similar situation is also observed for the cellular immune response against facultative intracellular tuberculosis and leprosy bacilli which themselves have relatively low levels of pathogenicity (Table 1). A healthy immune system will normally bring such infectious agents under control efficiently, and the immunological cell and tissue damage (which occurs in parallel with the elimination of the pathogen) will be minimal, ensuring that there is little by way of pathological or clinical consequence. However, should the immune system allow these agents to spread further, the result will be a chronic immunopathological response and resultant tissue destruction—as seen during hepatitis B as chronic or acute aggressive hepatitis and in leprosy as the tuberculoid form. Should a rapidly spreading infection result in exhaustion of the T cell response, or should an insufficient level of immunity be generated, the infected host will become a carrier. This carrier state, which only occurs during infections characterized by an absent or low- level of cytopathology, is convincingly demonstrated in hepatitis B carriers and sufferers of lepromatous leprosy.
Tumor Immunity
Our knowledge concerning the immune control of tumors is still modest. Some tumor types bear defined tumor-associated, or tumor-specific, antigens. However this is apparently not sufficient for induction of an efficient immune defense. There is also the problem of tumor diagnosis; the presence of tumors is sometimes confirmed using a functional or immunological basis, yet the tumor cannot be located because conventional examinations are often unable to discover them until they reach a size of about 109 cells (i.e., about 1 ml) of tumor tissue.
Factors important in immune defense reactions include the location and rate of proliferation, vascularization or the lack thereof, and necrosis with phagocytosis of disintegrating tumor tissue. We never actually get to see those rare tumors against which immune control might have been successfully elicited, instead we only see those clinically relevant tumors that have unfortunately become successful tumors which have escaped immune control. Evidence of the immune system's role in tumor control includes:
-Greater than 85 % of all tumors are carcinomas and sarcomas, that is non- lymphohematopoietic tumors which arise in the periphery, outside of organized lymphoid tissues. The immune system, in a manner similar to that seen for many strictly extra-lymphatic self antigens, ignores such tumors at first.
-Lymphohematopoietic tumors often present immunological oddities such as unusually low, or entirely absent, MHC and/or low tumor antigen concentrations, plus they frequently lack accessory molecules and signals.
-Congenital or acquired immunodeficiency—whether caused by anti-lymphocytic sera, cytostatic drugs, gamma irradiation, UV irradiation, or infection—usually encourages tumor growth, especially for lymphohematopoietic tumors. Carcinomas and sarcomas show little or no increased susceptibility. Interestingly, experimental carcinogens are frequently also immunosuppressive.
-Surgical removal of a large primary tumor may result in the disappearance (or rarely in rapid growth) of metastases within the lymph nodes.
-Tumor cells often display modulated MHC expression—some tumors lack MHC class I molecules entirely—or in some cases tumors selectively down- modulate the only MHC allele capable of presenting a specific tumor-associated peptide (e.g., the colon adenocarcinomas). Other tumors side-step immune defenses by down-regulating tumor-specific antigens.
-The immune response may fail if tumor differentiation antigens are expressed, against which the host exhibits an immunological tolerance (e.g., carcinoembryonic antigen [CEA], T-cell leukemia antigen).
-Blockade of the reticuloendothelial system may encourage the development of lymphohematopoietic tumors. For instance, chronic parasitic infections or infection by malaria can result in the development of Burkitt lymphoma, a B-cell malignancy.
References
Zinkernagel, R. M. (2005). Medical Microbiology.



|
|
|
|
للتخلص من الإمساك.. فاكهة واحدة لها مفعول سحري
|
|
|
|
|
|
|
العلماء ينجحون لأول مرة في إنشاء حبل شوكي بشري وظيفي في المختبر
|
|
|
|
|
|
|
قسم العلاقات العامّة ينظّم برنامجاً ثقافياً لوفد من أكاديمية العميد لرعاية المواهب
|
|
|