


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 22-11-2018
Date: 15-1-2018
Date: 16-12-2018
|
Extraction of the alkali metals
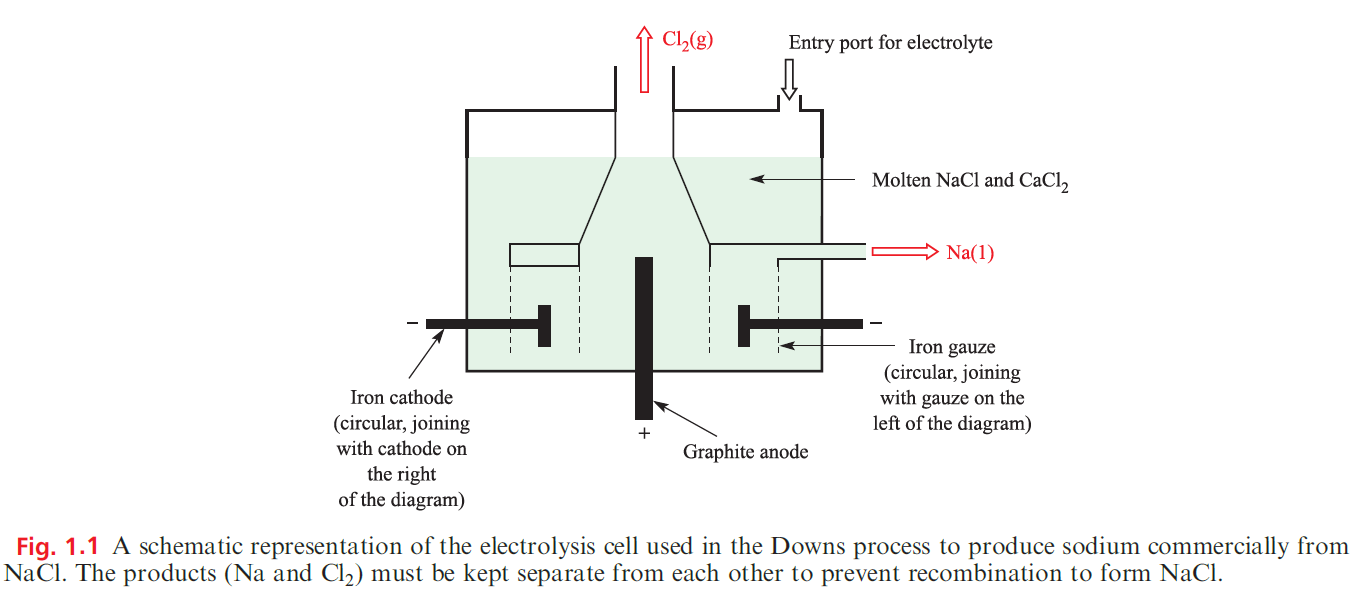
Sodium, economically much the most important of the alkali metals, is manufactured by the Downs process in which molten NaCl is electrolysed CaCl2 is added to reduce the operating temperature to about 870 K, since pure NaCl melts at 1073 K. The design of the electrolysis cell (Figure 1.1) is critical to prevent reformation of NaCl by recombination of Na and Cl2.
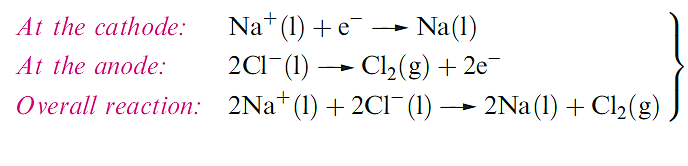
Lithium is extracted from LiCl in a similar electrolytic process; LiCl is first obtained from spodumene by heating with CaO to give LiOH, which is then converted to the chloride. Potassium can be obtained electrolytically from KCl, but a more efficient method of extraction is the action of Na vapour on molten KCl in a counter-current fractionating tower. This yields an Na–K alloy which can be separated into its components by distillation. Similarly, Rb and Cs can be obtained from RbCl and CsCl, small quantities of which are produced as by-products from the extraction of Li from spodumene. Small amounts of Na, K, Rb and Cs can be obtained by thermal decomposition of their azides an application of NaN3 is in car airbags.
Lithium cannot be obtained from an analogous reaction because the products recombine, yielding the nitride, Li3N .
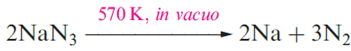



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|