


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 11-9-2017
Date: 3-9-2017
Date: 27-7-2017
|
OXIDATION OF PARAFFINS (Fatty Acids and Fatty Alcohols)
The catalytic oxidation of long-chain paraffins (Cl8-C30) over manganese salts produces a mixture of fatty acids with different chain lengths. Temperature and pressure ranges of 105–120°C and 15–60 atmospheres are used. About 60 wt% yield of fatty acids in the range of Cl2-Cl4 is obtained. These acids are used for making soaps. The main source for fatty acids for soap manufacture, however, is the hydrolysis of fats and oils (a nonpetroleum source). Oxidation of paraffins to fatty acids may be illustrated as:
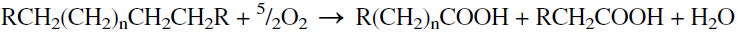
Oxidation of Cl2-Cl4 n-paraffins using boron trioxide catalysts was extensively studied for the production of fatty alcohols. Typical reaction conditions are 120–130°C at atmospheric pressure. ter-Butyl hydroperoxide (0.5%) was used to initiate the reaction. The yield of the alcohols was 76.2 wt% at 30.5% conversion. Fatty acids (8.9 wt%) were also obtained. Product alcohols were essentially secondary with the same number of carbons and the same structure per molecule as the parent paraffin hydrocarbon. This shows that no cracking has occurred under the conditions used. The oxidation reaction could be represented as:
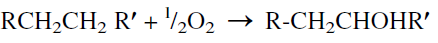
n-Paraffins can also be oxidized to alcohols by a dilute oxygen stream (3–4%) in the presence of a mineral acid. The acid converts the alcohols to esters, which prohibit further oxidation of the alcohols to fatty acids.
The obtained alcohols are also secondary. These alcohols are of commercial importance for the production of nonionic detergents (ethyoxylates):
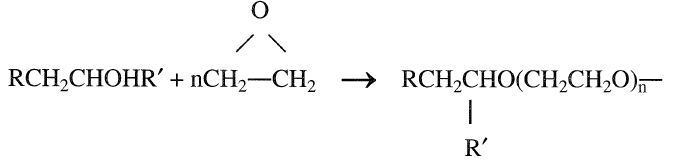



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
لحماية التراث الوطني.. العتبة العباسية تعلن عن ترميم أكثر من 200 وثيقة خلال عام 2024
|
|
|