
 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 9-10-2018
Date: 23-2-2019
Date: 16-12-2018
|
Other compounds of xenon
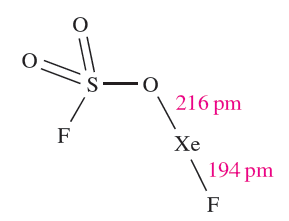
Members of a series of compounds of the type FXeA where, for example, A- is [OClO3]- , [OSO2F]-,[OTeF5]- or [O2CCF3]- have been prepared by the highly exothermic elimination of HF between XeF2 and HA. Further loss of HF leads to XeA2 (e.g. equation 1.1). Elimination of HF also drives the reaction of XeF2 with HN(SO3F)2 to yield FXeN(SO3F)2, a relatively rare example of Xe_N bond formation.
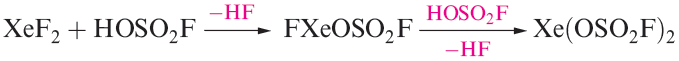 (1.1)
(1.1)
(1.1)
Xenon–carbon bond formation is now quite well exemplified, and many products contain fluorinated aryl substituents, e.g.(C6F5CO2)Xe(C6F5), [(2,6-F2C5H3N)XeC6F5] (Figure 1.1a), [(2,6-F2C6H3(Xe][BF4] (Figure 1.1b), [(2,6-F2C6H3)Xe][CF3SO3] and [(MeCN)Xe(C6F5)] .

Fig. 1.1 The structures (X-ray diffraction) of (a) [(2,6-F2C5H3N)Xe(C6F5)]+ in the [AsF6]- salt [H.J. Frohn et al. (1995) Z. Naturforsch., Teil B, vol. 50, p. 1799] and (b) [(2,6-F2C6H3)Xe][BF4] [T. Gilles et al. (1994) Acta Crystallogr., Sect. C, vol. 50, p. 411]. Colour code: Xe, yellow; N, blue; B, blue; C, grey; F, green; H, white.
The degree of interaction between the Xe centre and non-carbon donor (i.e. F, O or N) in these species varies. Some species are best described as containing Xe in a linear environment (e.g. Figure 1.1a) and others tend towards containing an [RXe]+ cation (e.g. Figure 1.1b). The compounds C6F5XeF and (C6F5)2Xe are obtained using the reactions in scheme 1.2. Stringent safety precautions must be taken when handling such compounds; (C6F5)2Xe decomposes explosively above 253 K.
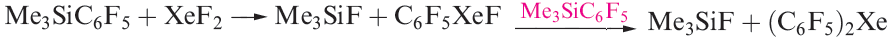 (1.2)
(1.2)
The [C6F5XeF2]+ ion (formed as the [BF4]- salt from C6F5BF2 and XeF4) is an extremely powerful oxidativefluorinating agent, e.g. it converts I2 to IF5. Compounds containing linear C_Xe_Cl units are recent additions to xenon chemistry, the first examples being C6F5XeCl (equation 1.3) and [(C6F5Xe)2Cl] (equation 1.4 and structure 1.2).
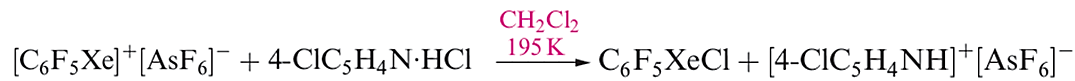 (1.3)
(1.3)
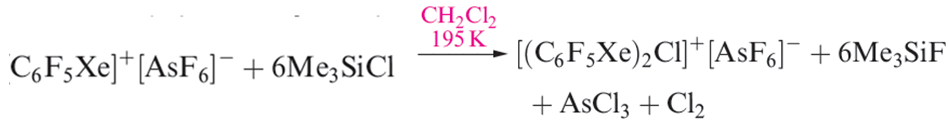 (1.4)
(1.4)
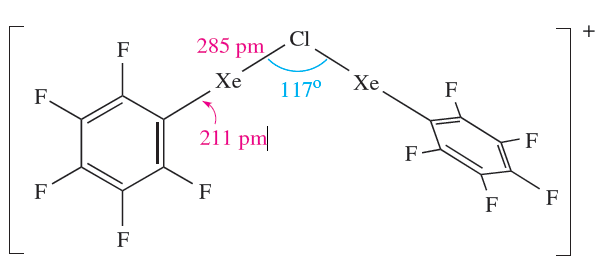
(1.2)
Compounds containing metal–xenon bonds have been known only since 2000. The first example was the square planar [AuXe4]2+ cation (av. Au_Xe = 275 pm). It is produced when AuF3 is reduced to Au(II) in anhydrous HF/SbF5 in the presence of Xe (equation 1.5).
 (1.5)
(1.5)
Removal of Xe from [AuXe4][Sb2F11]2 under vacuum at 195K leads to [cis-AuXe2][Sb2F11]2. The cis-description arises as a result of Au_F_Sb bridge formation in the solid state (diagram 1.3). The trans-isomer of [AuXe2]2+ is formed by reacting finely divided Au with XeF2 in HF/ SbF5 under a pressure of Xe, but if the pressure is lowered, the product is the Au(II) complex [XeAuFAuXe][SbF6]3.

(1.3)
The 2 oxidation state is rare for gold. The acid strength of the HF/SbF5 system can be lowered by reducing the amount of SbF5 relative to HF. Under these conditions, crystals of the Au(III) complex 1.4 (containing trans-[AuXe2F]2) are isolated from the reaction of XeF2, Au and Xe.
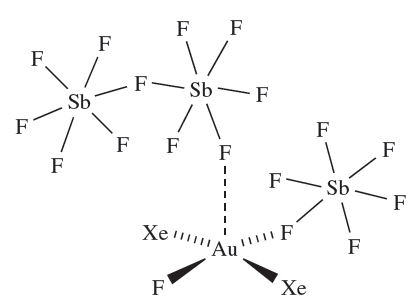
(1.4)



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
لحماية التراث الوطني.. العتبة العباسية تعلن عن ترميم أكثر من 200 وثيقة خلال عام 2024
|
|
|