


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 17-1-2018
Date: 27-4-2019
Date: 18-6-2019
|
Balancing charges: Magnesium and bromine
Suppose you want to know the formula, or composition, of a compound that results from reacting a metal and a nonmetal. You start by putting the two atoms side by side, with the metal on the left. Then you add their charges. Figure 1.1 shows this process for magnesium and bromine. (Forget about the crisscrossing lines for now. I explain them in the upcoming section “Using the crisscross rule.”)
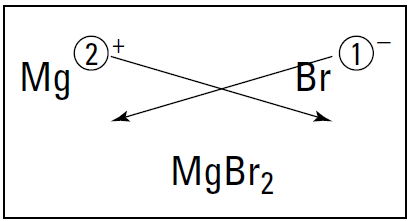
Figure 1.1: Figuring the formula of magnesium bromide.
The electron configurations for magnesium and bromine are
Magnesium (Mg): 1s22s22p63s2
Bromine (Br): 1s22s22p63s23p64s23d104p5
Magnesium, an alkaline earth metal, has two valence electrons that it loses to form a cation with a 2+ charge. The electron configuration for the magnesium cation is
Mg2+: 1s22s22p6
Bromine, a halogen, has seven valence electrons, so it gains one electron to complete its octet (eight valence electrons) and form the bromide anion with a 1– charge. The electron configuration for the bromide anion is
Br1–: 1s22s22p63s23p64s23d104p6
When writing the formula of a compound, the compound must be neutral. That is, it needs to have equal numbers of positive and negative charges. So after writing the atoms, you need to balance their charges.
The magnesium ion has a 2+, so it requires two bromide anions, each with a single negative charge, to balance the two positive charges of magnesium. So the formula of the compound that results from reacting magnesium with bromine is MgBr2.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|