


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 1-1-2017
Date: 1-1-2017
Date: 16-9-2020
|
Pressure, Temperature and RMS Speed
Now consider our first kinetic theory problem. Imagine a gas, consisting of n moles being confined to a cubical box of volume V. ''What is the connection between the pressure p exerted by the gas on the walls and the speeds of the molecules?" Pressure is defined as Force divided by Area or 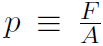 where
where 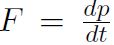 . Using Newtonian Mechanics, shows that
. Using Newtonian Mechanics, shows that

where n is the number of moles, M is the mass of 1 mole of the gas (so that nM is the total mass of the gas), vRMS is the average speed of the molecules and V is the volume of the gas. The above equation is derived purely from applying Newtonian mechanics to the individual molecules. Now by comparing to the ideal gas law pV = nRT or  we must have
we must have 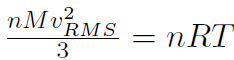 or
or

which shows that the temperature T is related to the speed of molecules!
As shown the speed of molecules at room temperature is very large; about 500 m/sec for air (about 1000 mph).



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|