
 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية | Crystal field stabilization energy: high- and low-spin octahedral complexes. |
|
|
|
Read More
Date: 23-2-2017
Date: 27-2-2017
Date: 23-6-2019
|
Crystal field stabilization energy: high- and low-spin octahedral complexes.
We now consider the effects of different numbers of electrons occupying the d orbitals in an octahedral crystal field. For a d1 system, the ground state corresponds to the configuration t2g1 (1.1). With respect to the barycentre, there is a stabilization energy of _ 0.4 Δoct (Figure 1.1); this is the so-called crystal field stabilization energy, CFSE. For a d2 ion, the ground state configuration is t2g2 and the CFSE = - 0:8 Δoct (equation 1.1); a d 3 ion (t2g3) has a CFSE= _1.2 Δoct.
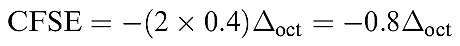 (1.1)
(1.1)
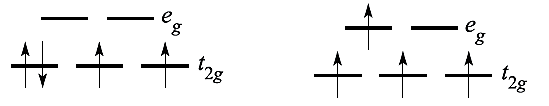
(1.1) (1.2)
For a d4 ion, two arrangements are available: the four electrons may occupy the t2g set with the configuration t2g4 (1.1), or may singly occupy four d orbitals, t2g3 eg1 (1.1).
Configuration 1.1 corresponds to a low-spin arrangement, and 1.2 to a high-spin case. The preferred configuration is that with the lower energy and depends on whether it is energetically preferable to pair the fourth electron or promote it to the eg level. Two terms contribute to the electron-pairing energy, P, which is the energy required to transform two electrons with parallel spin in different degenerate orbitals into spin-paired electrons in the same orbital:
For a given dn configuration, the CFSE is the difference in energy between the d electrons in an octahedral crystal field and the d electrons in a spherical crystal field . To exemplify this, consider a d4 configuration. In a spherical crystal field, the d orbitals are degenerate and each of four orbitals is singly occupied. In an octahedral crystal field, equation 20.3 shows how the CFSE is determined for a high-spin d4 configuration.
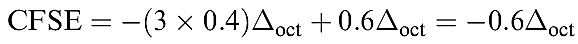 (1.2)
(1.2)
For a low-spin d4 configuration, the CFSE consists of two terms: the four electrons in the t2g orbitals give rise to a _1.6 Δoct term, and a pairing energy, P, must be included to account for the spin-pairing of two electrons. Now consider a d6 ion. In a spherical crystal field, one d orbital contains spin-paired electrons, and each of four orbitals is singly occupied. On going to the high-spin d6 configuration in the octahedral field (t2g4 eg2), no change occurs to the number of spin-paired electrons and the CFSE is given by equation 1.3.
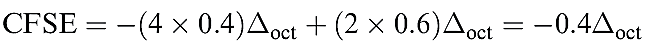 (1.3)
(1.3)
For a low-spin d6 configuration (t2g6 eg0) the six electrons in the t2g orbitals give rise to a _2.4 Δoct term.
Added to this is a pairing energy term of 2P which accounts for the spin pairing associated with the two pairs of electrons in excess of the one in the high-spin configuration. Table 1.1 lists values of the CFSE for all dn configurations. Inequality 1.4 holds when the crystal field is weak, whereas expression 1.5 is true for a strong crystal field. Figure 1.1 summarizes the preferences for low- and high-spin d5 octahedral complexes configurations in an octahedral crystal field. Inequalities 1.4 and 1.5 show the requirements for high- or low spin.
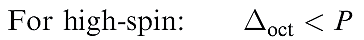 (1.4)
(1.4)
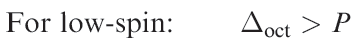 (1.5)
(1.5)
We can now relate types of ligand with a preference for high- or low-spin complexes. Strong field ligands such as [CN]- favour the formation of low-spin complexes, while weak field ligands such as halides tend to favour high-spin complexes. However, we cannot predict whether high- or low-spin complexes will be formed unless we have accurate values of Δoct and P. On the other hand, with some experimental knowledge in hand, we can make some comparative predictions: if we know from magnetic data that [Co(H2O)6]3 is low-spin, then from the spectrochemical series we can say that [Co(ox)3]3- and [Co)CN(6]3- will be low-spin. The only common high-spin cobalt(III) complex is [CoF6]3-.
Table 1.1 Octahedral crystal field stabilization energies (CFSE) for d n configurations; pairing energy, P, terms are included whereappropriate. High- and low-spin octahedral complexes are shown only where the distinction is appropriate.
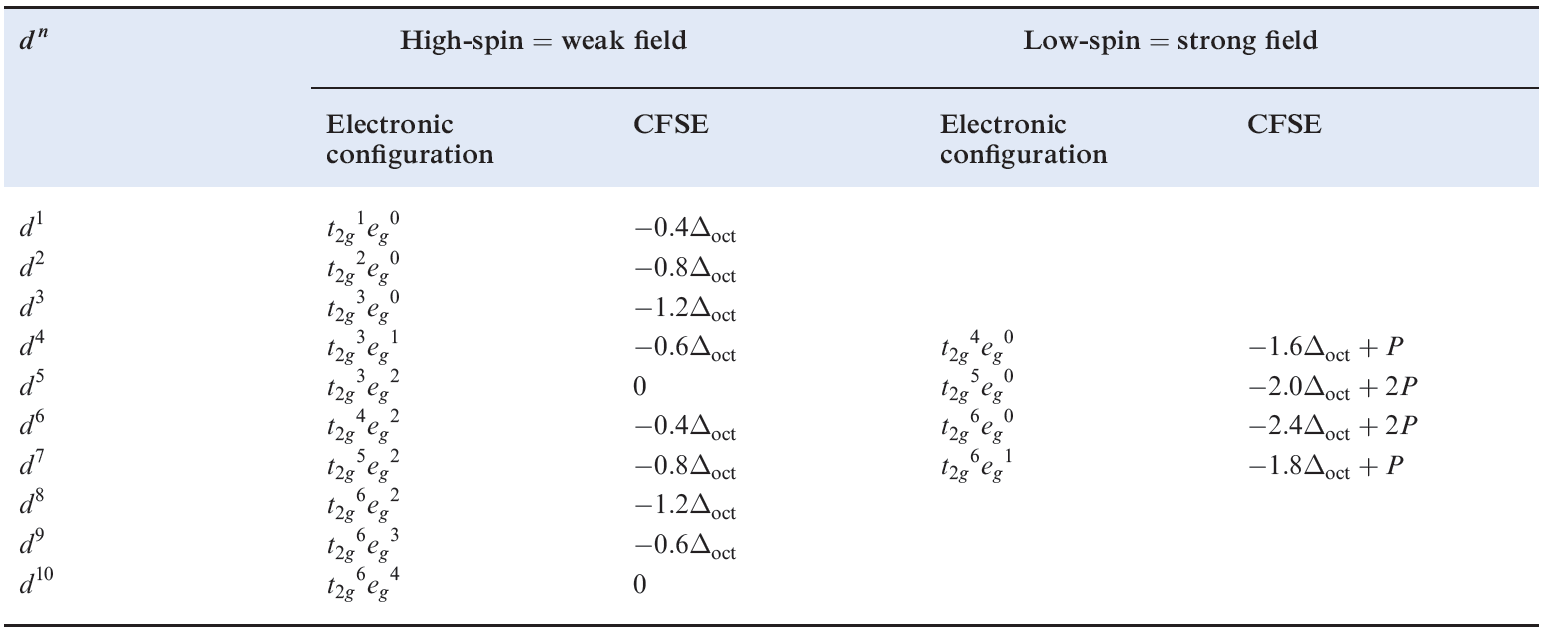
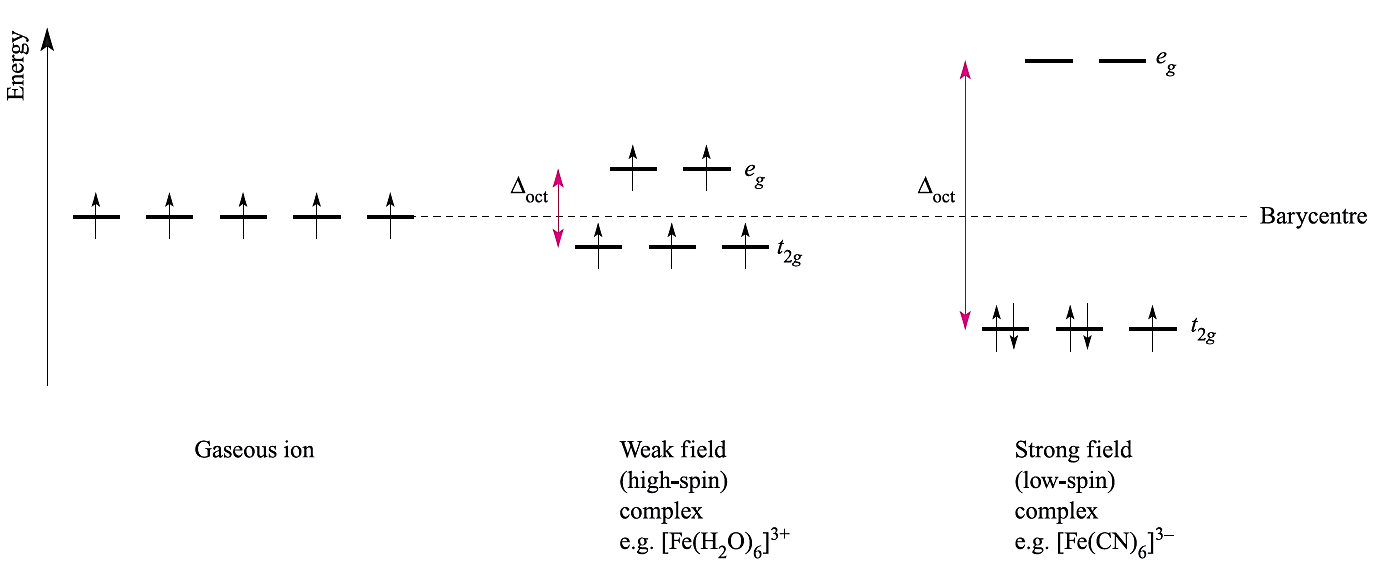
Fig. 1.1 The occupation of the 3d orbitals in weak and strong field Fe3+ (d5) complexes.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|