


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 21-9-2020
Date: 12-4-2016
Date: 8-7-2018
|
Reduction of Alkenes: Hydrogenation
Alkenes react with H2 in the presence of a metal catalyst such as palladium or platinum to yield the corresponding saturated alkane addition products. We describe the result by saying that the double bond has been hydrogenated, or reduced. Note that the word reduction is used somewhat differently in organic chemistry from what you might have learned previously. In general chemistry, a reduction is defined as the gain of one or more electrons by an atom. In organic chemistry, however, a reduction is a reaction that results in a gain of electron density for carbon, caused either by bond formation between carbon and a less electronegative atom—usually hydrogen—or by bond-breaking between carbon and a more electronegative atom—usually oxygen, nitrogen,or a halogen.. Reduction Increases electron density on carbon by:
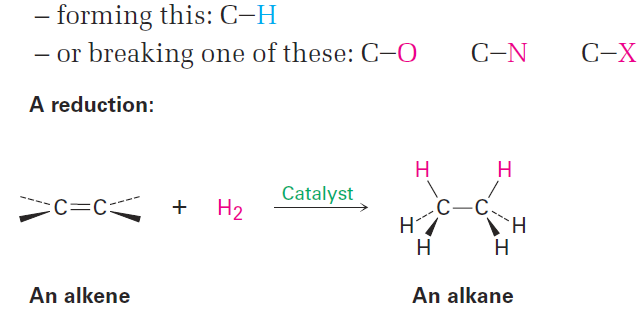
Platinum and palladium are the most common laboratory catalysts for alkene hydrogenations. Palladium is normally used as a very fine powder “supported” on an inert material such as charcoal (Pd/C) to maximize surface area. Platinum is normally used as PtO2, a reagent known as Adams’ catalyst after its discoverer, Roger Adams.
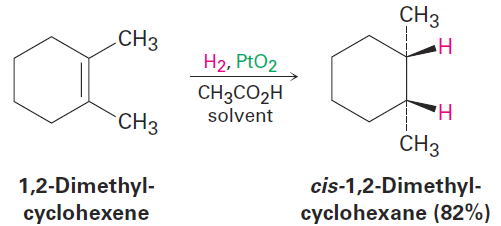
Catalytic hydrogenation, unlike most other organic reactions, is a heterogeneous process rather than a homogeneous one. That is, the hydrogenation reaction does not occur in a homogeneous solution but instead takes place on the surface of solid catalyst particles. Hydrogenation usually occurs with syn stereochemistry: both hydrogens add to the double bond from the same face.
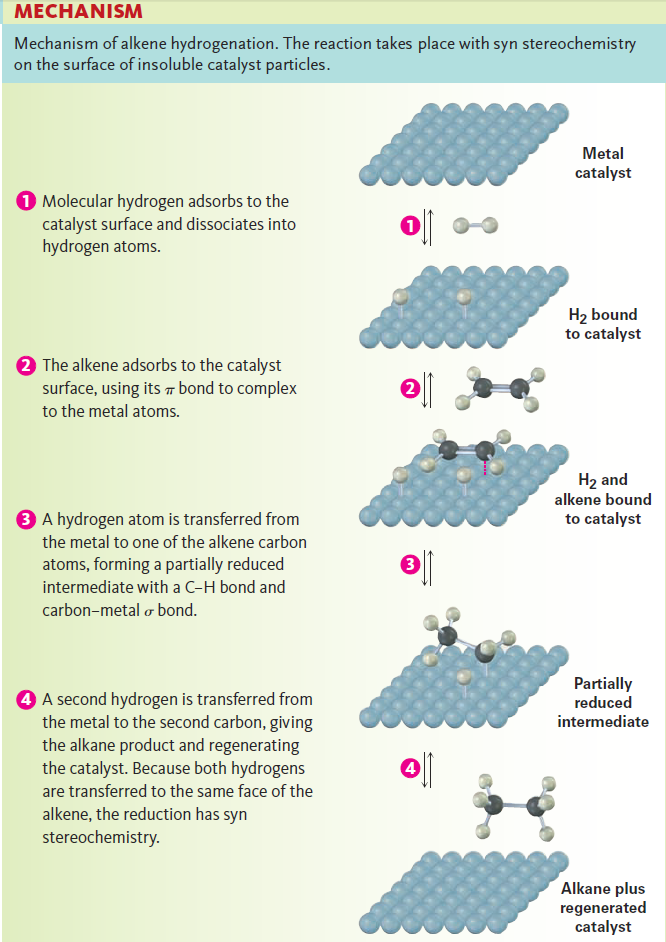
As shown mechanism below, hydrogenation begins with adsorption of H2 onto the catalyst surface. Complexation between catalyst and alkene then occurs as a vacant orbital on the metal interacts with the filled alkene p orbital. In the final steps, hydrogen is inserted into the double bond and the saturated product diffuses away from the catalyst. The stereochemistry of hydrogenation is syn because both hydrogens add to the double bond from the same catalyst surface.
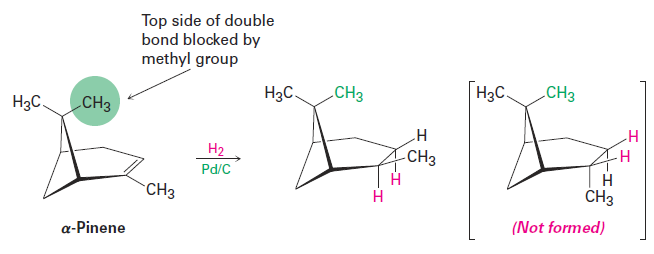
An interesting feature of catalytic hydrogenation is that the reaction is extremely sensitive to the steric environment around the double bond. As a result, the catalyst usually approaches the more accessible face of an alkene, giving rise to a single product. In a-pinene, for example, one of the methyl groups attached to the four-membered ring hangs over the top face of the double bond and blocks approach of the hydrogenation catalyst from that side. Reduction therefore occurs exclusively from the bottom face to yield the product shown.
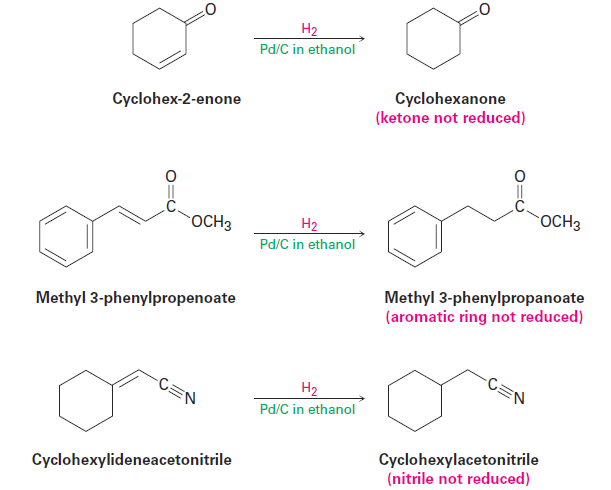
Alkenes are much more reactive toward catalytic hydrogenation than most other unsaturated functional groups, and the reaction is therefore quite selective. Other functional groups, such as aldehydes, ketones, esters, and nitriles, often survive alkene hydrogenation conditions unchanged, although reaction with these groups does occur under more vigorous conditions. Note that, particularly in the hydrogenation of methyl 3-phenylpropenoate shown below, the aromatic ring is not reduced by hydrogen and palladium even though it contains apparent double bonds.
In addition to its usefulness in the laboratory, catalytic hydrogenation is also important in the food industry, where unsaturated vegetable oils are reduced on a large scale to produce the saturated fats used in margarine and cooking products (Figure 1-1).
The fatty acids are generally polyunsaturated, and their double bonds have cis stereochemistry. Complete hydrogenation yields the corresponding saturated fatty acids, but incomplete hydrogenation often results in partial cis–trans isomerization of a remaining double bond. When eaten and digested, the free trans fatty acids are released, raising blood cholesterol levels and potentially contributing to coronary problems.

Figure 1-1 Catalytic hydrogenation of polyunsaturated fats leads to saturated products, along with a small amount of isomerized trans fats.
Double-bond reductions are extremely common in biological pathways, although the mechanism is of course different from that of laboratory catalytic hydrogenation over palladium. As with biological hydrations, biological reductions usually occur in two steps and require that the double bond be adjacent to a carbonyl group. In the first step, the biological reducing agent NADPH (reduced nicotinamide adenine dinucleotide phosphate), adds a hydride ion (H:-) to the double bond to give an anion. In the second, the anion is protonated by acid HA, leading to overall addition of H2. An example is the reduction of trans-crotonyl ACP to yield butyryl ACP, a step involved in the biosynthesis of fatty acids (Figure 1-2).

Figure 1-2 Reduction of the carbon–carbon double bond in trans-crotonyl ACP, a step in the biosynthesis of fatty acids. One hydrogen is delivered from NADPH as a hydride ion, H:-; the other hydrogen is delivered by protonation of the anion intermediate with an acid, HA.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|