

 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 21-8-2016
Date: 20-1-2020
Date: 5-7-2019
|
Properties of Alkanes
Alkanes are sometimes referred to as paraffins, a word derived from the Latin parum affinis, meaning “little affinity.” This term aptly describes their behavior, for alkanes show little chemical affinity for other substances and are chemically inert to most laboratory reagents. They are also relatively inert biologically and are not often involved in the chemistry of living organisms. Alkanes do, however, react with oxygen, halogens, and a few other substances under appropriate conditions. Reaction with oxygen occurs during combustion in an engine or furnace when an alkane is used as a fuel.

Carbon dioxide and water are formed as products, and a large amount of heat is released. For example, methane (natural gas) reacts with oxygen according to the equation The reaction of an alkane with Cl2 occurs when a mixture of the two is irradiated with ultraviolet light (denoted hυ, where υ is the Greek letter nu).
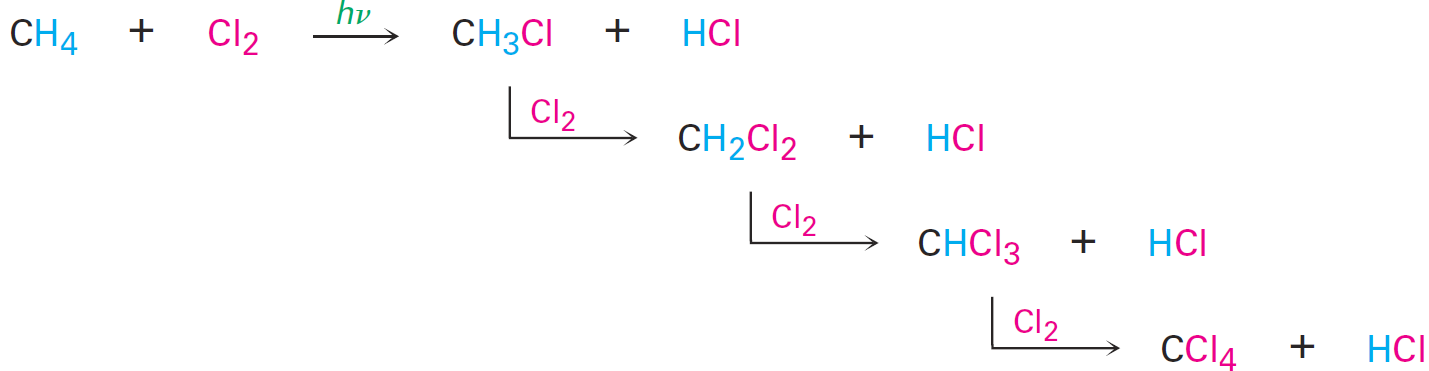
Depending on the time allowed and the relative amounts of the two reactants, a sequential substitution of the alkane hydrogen atoms by chlorine occurs, leading to a mixture of chlorinated products. Methane, for instance, reacts with Cl2 to yield a mixture of CH3Cl, CH2Cl2, CHCl3, and CCl4.
Alkanes show regular increases in both boiling point and melting point as molecular weight increases (Figure1), an effect due to the presence of weak dispersion forces between molecules. Only when sufficient energy is applied to overcome these forces does the solid melt or liquid boil. As you might expect, dispersion forces increase as molecule size increases, accounting for the higher melting and boiling points of larger alkanes.

Figure 1: A plot of melting and boiling points versus number of carbon atoms for the
C1–C14 straight-chain alkanes. There is a regular increase with molecular size.
Another effect seen in alkanes is that increased branching lowers an alkane’s boiling point. Thus, pentane has no branches and boils at 36.1°C, isopentane (2-methylbutane) has one branch and boils at 27.85 °C, and neopentane (2,2-dimethylpropane) has two branches and boils at 9.5 °C. Similarly, octane boils at 125.7 °C, whereas isooctane (2,2,4 trimethylpentane) boils at 99.3 °C. Branched-chain alkanes are lower boiling because they are more nearly spherical than straight-chain alkanes, have smaller surface areas, and consequently have smaller dispersion forces.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|