


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 19-4-2016
Date: 17-4-2016
Date: 18-4-2016
|
SDS-PAGE
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was introduced in 1967 by Shapiro and has since become one of the most popular PAGE methods. The method is dependent in the first instance upon the interaction of the protein molecule with SDS. This is a detergent having a 12 carbon hydrophobic tail and a hydrophilic, sulfonic acid head group (I).

Proteins are denatured by boiling in the presence of SDS. The SDS molecules interact with the proteins to give rod-like complexes, containing a constant ratio of ca. 1.4 mg of SDS per mg of protein. At this level the negative charge on the SDS is sufficient to mask the charge on the protein and all proteins consequently have essentially the same charge/mass ratio and an anodic migration.
The lack of charge differences between different protein/SDS complexes means that the stacking phase will not be as effective as in conventional disc electrophoresis. However, Laemmli has devised a convenient method whereby disc PAGE and SDS-PAGE can be conducted with the same set of reagents, simply with or without SDS. The Laemmli method has become one of the most popular PAGE methods in use today. However, the Laemmli method does not separate small proteins very well and an alternative SDS-PAGE method, using Tricine buffer, has been described by Shagger and Von Jagow. The Tricine method gives uniquely good separation of proteins under 20 kDa. As the intrinsic charge differences between proteins is masked by the SDS, separation of proteins is due solely to differences in size and hence the method can be used to determine molecular sizes. A linear relationship between mobility and log MW obtains over a molecular weight range dependent upon the gel pore size. The gel can thus be standardized with proteins of known molecular weight and subsequently used to estimate the molecular weights of unknowns. Mobility is conveniently expressed as relative mobility (Rm), i.e. mobility relative to the bromophenol blue tracker dye.
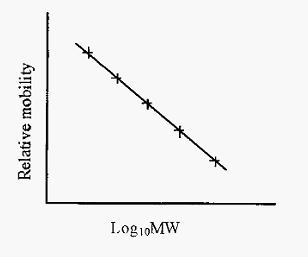
Figure 1. Standard curve for estimation of protein MWs by SDS-PAGE.
Some caveats apply to the estimation of molecular weights. If proteins are not reduced, then disulfide bridges may constrain the structure and prevent formation of the rod-like complexes. This will result in an incorrect apparent MW. On the other hand, this provides a way of detecting the existence of disulfide bridges. The glyco moiety of glycoproteins will also not bind SDS, and yet will contribute to the steric resistance of the molecule. In consequence, the molecular weights of glycoproteins tend to be overestimated. Finally, boiling in SDS not only denatures the protein but will dissociate the subunits of oligomeric proteins. The MWs obtained by SDS-PAGE will therefore be of the subunits and not of the intact protein.
An SDS-PAGE zymogram for proteinases
Zymography is a method for the detection of a specific enzyme among the bands separated by electrophoresis. The method usually relies on an enzyme specific reaction to generate a colour to reveal the position of the enzyme. Usually zymogram methods cannot be applied to SDS-PAGE as proteins are normally denatured in this technique.
However, Heussen and Dowdle have devised an ingenious SDS-PAGE zymogram method for proteinases. The method depends upon combining the proteinase-containing mixture with SDS, but without boiling the solution. In this form the SDS can apparently combine only partially with the protein, perhaps “tweaking” its conformation slightly and suppressing the activity of proteinases.
The SDS/protein mixture is separated by electrophoresis in a polyacrylamide gel containing a low concentration of gelatin (less than 1%). The proteinase/SDS complex does not bind to the gelatin, as would a free proteinase, and migrates as a narrow band. Subsequent incubation in a non-ionic detergent, such as Triton X-100, removes the SDS from the proteinase and reconstitutes its activity. The reactivated proteinase digests away part of the gelatin and its position can be detected by subsequently staining the gel. The proteinase-digested gelatin appears as a clear band on the blue stained gel.
References
Dennison, C. (2002). A guide to protein isolation . School of Molecular mid Cellular Biosciences, University of Natal . Kluwer Academic Publishers new york, Boston, Dordrecht, London, Moscow .
Shapiro, A. L., ViÒuela, E. and Maizel, J. V. Jnr (1967) Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem. Biophys. Res. Commun. 28, 815-820.
Weber, K. and Osborn, M. (1969) The reliability of molecular weight determinations by dodecyl-sulphate-polyacrylamide gel electrophoresis. J. Biol. Chem. 244, 4406-4412.
Shgger, H. and von Jagow, G. (1 987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1-100 kDa. Anal. Biochem. 166, 368-379.
Heussen, C. and Dowdle, E. B. (1996) Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal. Biochem. 102, 196-202.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
قسم شؤون المعارف ووفد من جامعة البصرة يبحثان سبل تعزيز التعاون المشترك
|
|
|