


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 24-10-2018
Date: 13-3-2019
Date: 22-11-2018
|
Exchange energies
Filled and half-filled shells are often referred to as possessing a ‘special stability’. However, this is misleading, and we should really consider the exchange energy of a given configuration. This can only be justified by an advanced quantum mechanical treatment but we can summarize the idea as follows. Consider two electrons in different orbitals. The repulsion between the electrons if they have anti-parallel spins is greater than if they have parallel spins, e.g. for a p2 configuration:

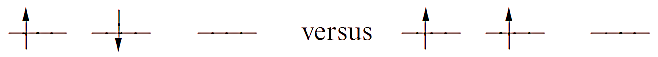
The difference in energy between these two configurations is the exchange energy, K, i.e. this is the extra stability that the right-hand configuration has with respect to the left-hand one. The total exchange energy is expressed in terms of K (the actual value of K depends on the atom or ion):

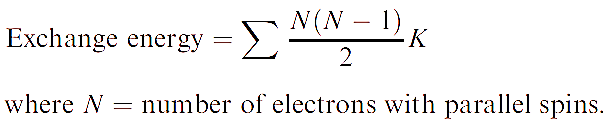
For further discussion, see:
A.B. Blake (1981) Journal of Chemical Education, vol. 58,
p. 393. B.J. Duke (1978) Education in Chemistry, vol. 15, p. 186.
D.M.P. Mingos (1998) Essential Trends in Inorganic Chemistry, Oxford University Press, Oxford, p. 14.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|