


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 2-5-2021
Date: 18-6-2021
Date: 19-12-2015
|
Detergents
1. Detergents as Amphiphilic Molecules
Gene expression studies in both eukaryotes and prokaryotes eventually require detecting and enriching the expression product, usually a protein. When this gene product is a water-soluble, cytosolic protein, detecting and enriching it can begin by simply homogenizing the cells, followed by centrifugally separating the supernatant from the membrane-bound proteins that remain in the pellet. When the gene product is a plasma membrane protein, however, the task is complicated because the protein has to be detached from the membrane at some stage. Although some loosely attached proteins are released by treatment with salt solutions, integral membrane proteins are released only upon treatment with amphiphilic reagents, also termed detergents .
By definition, amphiphiles harbor both hydrophilic and hydrophobic moieties. In a water-oil or water-lipid mixture, detergent molecules accumulate at the interface. The hydrophobic arm is inserted into oil or lipid, and the hydrophilic (polar) end is introduced into water, thus justifying the adjective “amphiphilic” used to describe this class of compounds. Depending on the degree of polarity, the hydrophilic end (the polar head group) of a detergent is either ionic or nonionic. Regardless of the difference in polar head groups, all detergents dissolve in water. The physicochemical properties of detergents dissolved in water have been the focus of vigorous biochemical research.
Physiological Importance of Detergents
Amphiphilic substances or detergents are central to our existence as living organisms. About 80% of the lipids in the alveolar lining is dipalmitoyl phosphatidylcholine that acts as a detergent to confer the crucial nonatelectic property to the lungs. This unusual proportion of a particular phospholipid is important because lower levels of this lipid in the alveolus lead to atelectasis (collapsed alveolus) in infants. Similarly, bile acids are crucial detergent molecules in the intestines. Bile acids emulsify water-insoluble fat molecules and thus help the intestinal lining to absorb them. The powerful detergents, lysophospholipids, produced in the intestine by phospholipase A2-mediated cleavage of the C2 fatty acid chains of phospholipids, also aid in lipid digestion. In fact both bile acids and lysophospholipids aid in lipid absorption and also help in absorbing and removing the metabolic products of fats and lipids.
2. General Strategy for Obtaining Solubilized and Reconstituted Membrane Proteins
Solubilization of a membrane protein is a procedure in which the proteins and lipids, originally cradled in the membrane, are dissociated appropriately in a buffered detergent solution. This controlled dissociation of the membrane results in the forming small protein and lipid clusters that remain dissolved in the aqueous solution. Next the solubilized material is separated from any insoluble substance (aggregated proteins and lipids that are dissociated by the detergent) by centrifugation. Then, the detergent is removed from the solubilized material because only after removing the detergent do the solubilized proteins and lipids reaggregate appropriately so that the activity of a functional protein is restored. Many of the functional membrane proteins retain their activity only when they are allowed to associate with other specific membrane proteins and lipids. Such reaggregation is inhibited in the presence of the dissociation-promoting detergent. Typical methods to remove detergents include (1) extensive dialysis of the detergent-solubilized fractions, whereby the detergent monomers are released from the dialysis bag; (2) gel filtration, which helps to retain the detergent monomers on the column while the high molecular weight, solubilized proteins flow through; or (3) hydrophobic chromatography, whereby the hydrophobic detergent molecules are removed by absorption into a hydrophobic matrix. The success of each procedure depends on the physicochemical properties of the detergent, which determine the formation of detergent aggregates, termed micelles (Fig. 1). Therefore, it is important to identify and define some of the factors that regulate aggregation of detergent molecules. The critical micelle concentration ) cmc) is the maximum concentration at which the detergent molecules remain as monomers. Removal of detergents by dialysis or gel filtration occurs through the monomer and is difficult when the cmc is very low.

Figure 1. Structures of detergents and micelles. At the critical micelle concentration (cmc), monomeric molecules (a), which are stable below cmc and removable by dialysis, aggregate to form a spherical or cylindrical macromolecular micelle (b), which is stable above the cmc and cannot be removed by dialysis. In a micelle, the hydrophobic ends of the detergent molecules are pointed inward and away from water whereas the polar ends (head groups) are pointed outward into the aqueous phase. In the presence of a plasma membrane bilayer containing saturated and unsaturated fatty acid containing phospholipids (c), mixed micelles (d) that contain detergent molecules and plasma membrane lipids and proteins are formed.
2.1. Different Classes of Detergents and Their Applicability in Solubilizing Membrane Proteins As shown in Fig. 2, the various detergents can be structurally classified as follows:
1. Negatively charged, strongly ionic detergents: (a) a detergent with a long, flexible aliphatic chain: sodium dodecylsulfate (SDS) (cmc ~0.23% (w/v) or 8 mM); (b) a detergent with a rigid structure: sodium cholate (cmc ~0.43% or 10 mM). Sodium cholate (1%) has been used to solubilize the adenylate cyclase-G-protein complex (1), the dopamine D1 receptor (2) and many other receptors.
2. Detergents that form hydrogen bonds: (a) detergents with flexible long-chain structures: octanoyl- N-methylglucamide (MEGA-8) (cmc ~1.9% or 60 mM), and a long chain polyoxyether, Thesit (cmc ~0.005% or 0.09 mM), which is structurally similar to Lubrol. Although 0.5% MEGA-8 has been used to solubilize intrinsic membrane proteins (3), a Thesit concentration of 1.2% has been used with membrane proteins, such as adenylate cyclase (4). (b( more polar but non-ionic detergents with sugar rings, n-dodecyl-ß-maltoside (cmc ~0.009% or 0.18 mM) and n-octyl-ß-glucoside (cmc ~0.7% or 23.2 mM), which have been used to solubilize the nicotinic acetylcholine receptor [1% n-octyl-ß-D-glucoside, (5)], opioid receptors [0.3% n-octyl-ß-D-glucoside, (6)], GABAA receptors [1% n-octyl-ß-D-glucoside, (7)], photoreceptor guanylate cyclase [1% n-dodecyl-ß-D-maltoside, (8)], and others.
3. Hydrophobic detergents with long polyoxyether chains and aromatic rings: Triton X-100 (cmc ~0.013% or 0.2 mM) and Triton X-114 (cmc ~0.011% or 0.21 mM), which are used to solubilize the receptors for GABA [2% Tr X-100, (9)], prostacyclin (10), prolactin [1% Tr X-100, (11)], transferrin [1% Tr X-100, (12)], insulin [2% Tr X-100, (13)] and a neuronal growth cone protein, GAP-43 [1% Tr X-114, (14)].
4. Zwitterionic detergents: (a) a detergent with a flexible, long-chain structure: N-dodecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (in short, propanesulfonate) (cmc ~0.12% or 3.6 mM); (b) detergents with a rigid structure: CHAPS (cmc ~0.46% or 7.4 mM) and CHAPSO (cmc 8,~0.5% mM), commonly used to solubilize receptors, such as those for colony-stimulating factor [0.5% CHAPS, (15)], opiates [0.6% CHAPS, (16)], neurotensin [0.6% CHAPS, (17)], somatostatin [0.6% CHAPS, (18)], adenosine A1 [2.5% CHAPS, (19)] and human prostatic sex-hormone-binding globulin receptor [6.0% CHAPS, (20)].
5. Positively charged, strongly ionic detergents, for example, cetylpyridinium chloride.
6. Nonionic surfactants that form inclusion complexes, for example, cyclodextrin.
7. Specific detergents, such as digitonin, used to solubilize opioid receptors [2% digitonin, (21)], neurotensin receptors [2% digitonin, (22)], ß-adrenoreceptors (23), dihydropyridine Ca2+ channels [1% digitonin, (24)], and many others.
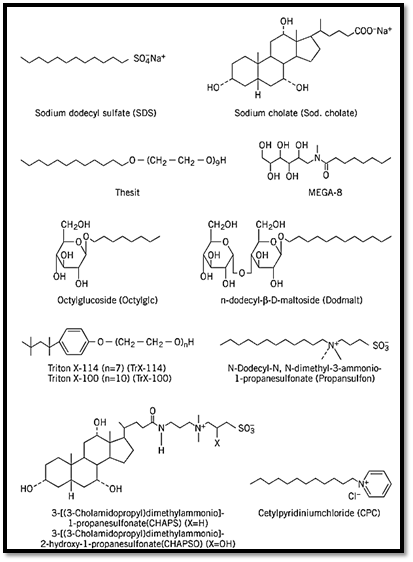
Figure 2. Classification of detergents based on structural features.
Thus, the detergent concentrations used for solubilizing most biologically active receptor proteins are typically in the range of 0.5 to 2.0% (w/v), suggesting that for practical purposes the cmc is not the deciding factor for choosing detergent concentration. Instead, it is obtained empirically by optimizing protein-solubilization conditions.
The other key factor in solubilizing biologically active membrane proteins is the ratio of detergent to protein maintained during solubilization. The commonly used ratios are 1:1 (16, 19) and 2 to 3:1 (4, 7,8) . Higher ratios, such as 10:1 (20) are used less often. Optimization experiments observed that the coextracted lipids are essential for reconstituting the solubilized, sheep brain serotonin 5-HT1A receptor (25), and the highest yield of the active 5-HT 1A sites was obtained with 2% CHAPS at a detergent to protein ratio of 2:1 (26). This topic is discussed in more detail later.
2.2. Other Uses of Detergents
Detergents are also used in purifying DNA and RNA molecules. For example, SDS is used to denature polynucleotide molecules during the preparation of DNA, and a structurally related detergent, sodium salt of N-laurylsarcosine (Sarkosyl), is used in preparing RNA (27, 28). In either case, the detergent is used to denature the biopolymers and also to inhibit certain enzymes, such as deoxyribonucleases and ribonucleases, which degrade these molecules. Thus detergents are inseparable from scientific activity whether it is to solubilize lipids and proteins or to purify DNA and RNA.
2.3. Detergent Solubilization of Membrane Lipids
A survey of the literature (1-26) indicates that there is no general agreement on the choice of detergent for a single membrane protein, and the use of five or more different classes of detergents for a given receptor solubilization, usually at a concentration 1 to 2%, irrespective of the cmc, is quite common. This could account for the variable recovery of biological activity in different preparations, but it does not explain the differential efficacy of the different detergents in solubilizing active receptor proteins.
To resolve this question, the protein- and lipid-extracting abilities of each detergent in Fig. 2 were determined. Sufficient variability was observed to explain much of the bewildering variability in the recovery of biological activity reported in the literature (26). The receptor molecule used as a marker in this study was the sheep brain serotonin 5-HT 1A receptor, which is a member of the receptor family with seven transmembrane regions, and may couple either to adenylate cyclase or phospholipase C, depending on the type of cell in which it is expressed (26). Solubilized, high-affinity serotonin 5-HT1A receptor sites are reconstituted only by coextracted lipids, and adding separately isolated, pure or mixed lipids during or after solubilization is completely ineffective in reactivating an inactive preparation (25). Instead, adding lipids or lipid vesicles to already solubilized, reconstituted (in coextracted lipids) and active 5-HT1A-R preparations inhibits the ligand-binding activity of the receptor. Such findings indicated that coextraction of plasma membrane lipids is important in order to maintain the functionally active conformation of lipid-sensitive membrane proteins, such as the serotonin 1A receptor. So the efficiency of many detergents in solubilizing membrane lipids was studied. Considering the total amount of lipid solubilized by each detergent, detergents with long aliphatic chains that terminate in a negative charge (SDS), a zwitterionic sequence (CHAPS or CHAPSO), or a multihydroxylic-group-containing disaccharide ( n-dodecyl-b-D-maltoside) (DDM) clearly has greater ability to solubilize lipids, irrespective of the cmc of the detergent (Fig. 3). In contrast to detergents, such as sodium cholate or sodium deoxycholate, which are also efficient in solubilizing membrane lipids, CHAPS, CHAPSO and DDM solubilized active 5-HT1A receptors. These results indicate that CHAPS, CHAPSO, and DDM, which are relatively milder and nondenaturing, allow the 5-HT1A-R-membrane lipid complex to remain undisturbed during solubilization, thus allowing recovery of the active 5-HT1A-R following removal of these detergents (25, 26).
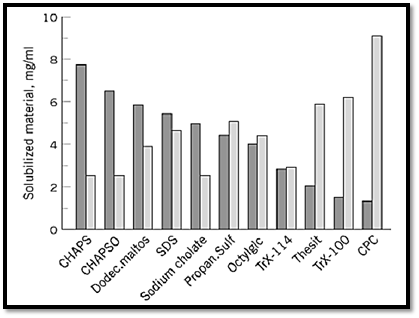
Figure 3. Concentration of proteins and lipids (mg/mL) in the detergent-solubilized and reconstituted vesicles, compared to the solubilization of high-affinity 5-HT1A binding sites.
Sodium cholate has a high cmc of approximately 8 mM (or 0.5%) and is easily dialyzed like CHAPS or CHAPSO (both of which have a cmc of ~0.5%). However, although it forms vesicles (because it coextracts sufficient lipid to give a similar lipid to protein ratio of ~2:1) (Table 1 and Fig. 3), the 5-HT1A receptors obtained in the reconstituted preparations are inactive. Possible explanations include nonextraction or denaturation of the protein because of the strongly ionic nature of sodium cholate. Octylglucoside presents a different picture. As a mild, nonionic, dialyzable (cmc ~0.7%) and very mildly denaturing detergent, it is often used to extract biologically active proteins (5-7). However, its inability to coextract sufficient lipid (a lipid to protein ratio of 1:1.1) probably accounts for the low amount of solubilized 5-HT1A receptor activity, which contains less than 15% of the lipids in comparable DDM supernatants.
Table 1. Distribution of Lipids, Protein, and Serotonin 5HT1A Receptor Binding Activity in the Vesicles Obtained after Detergent Removal Followed by Dialysis and Ultracentrifugation
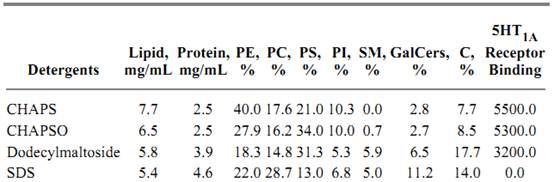
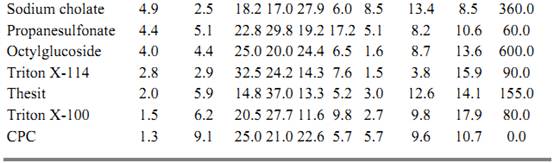
The detergents that extract large amounts of protein but are at the lower end of the lipid extraction scale (such as Thesit, Triton X-114, Triton X-100, and CPC) cannot solubilize enough lipid to produce vesicles and do not solubilize active 5-HT1A sites. Therefore, such G-protein-associated receptors require an environment containing particular lipids (enriched in PE, PS and PI) to express their biological properties fully.
The relative amounts of individual groups of lipids solubilized by the different classes of detergents are surprisingly diverse (Table 1). The inverse profiles of PC and PS are intriguing in view of earlier work showing that PC [and GalCer, sulfated GalCer (Su) and SM] are enriched in the outer membrane domain, whereas PS (and PE and PI) are enriched in the inner membrane domain (29-31). From the profile for SM it also appears that this lipid is probably localized in the PC-containing domains. This is agrees well with the idea that choline phospholipids are concentrated in the outer leaflet of the membrane bilayer. It is hard to explain this asymmetrical extraction of lipids in terms other than specific detergent binding by the protein. Thus for eight of the twelve detergents tested, it was found that the solubilization-enrichment profile of PI is similar to that of PE, indicating a similar, inner membrane localization of PE, PI, and PS. PS binds to protein kinase C on the cytosolic face of the plasma membrane and results in activating this enzyme (31). This is consistent with the idea that the PI family is the source of internally released second messengers (32). The serotonin 5-HT1A receptor polypeptide has seven transmembrane alpha-helices and three cytosolic loops (26), which could be more strongly associated with inner membrane lipids. Differences in protein folding could explain why a strongly denaturing detergent, such as sodium cholate, is useful in solubilizing and reconstituting the dopamine D1 receptor [which can be reconstituted by adding separately isolated lipids (2)] but is ineffective in solubilizing and reconstituting the 5-HT1A receptors.
3. Summary
The importance of detergents in the normal functions of a living body is irrefutable. In scientific research, detergents provide a powerful means of undertaking subcellular fractionation of macromolecules, such as proteins, RNA, and DNA. Although gene expression studies do not directly begin with protein purification, the expressed protein is finally purified or detected through biochemical methods, such as column chromatography, Western blotting analysis, or enzyme assays. If the expressed protein is lysosomal or cytosolic, simply homogenizing the cells in a hypotonic buffer, followed by separation of the soluble proteins by centrifugation, yields a supernatant that contains the required protein. However, if the expressed protein is, for example, a mitochondrial membrane protein, detergent solubilization of this protein is required before its assay. Although in earlier studies such choices of detergents were made empirically, the data presented here provide a stronger foundation for judicious analysis of the efficacy and applicability of many of the commonly used detergents. Thus, for immunoblot analysis, one might use Triton X-100 or Nonidet P-40. To solubilize proteins that do not require membrane lipids for acquiring functional activity, one might use either the Triton family of detergents, the bile acid detergents (eg, sodium cholate), or octylglucoside. On the other hand, if the protein is heavily lipid-dependent, one should test CHAPS, CHAPSO, or DDM before using other detergents. Therefore, this practical overview of the structural classification of detergents with appropriate reference to their applicability to protein and lipid solubilization and to reconstitution of functional activity could be helpful in scientific research involving expression of specific genes.
References
1. P. C. Sternweis and A. G. Gilman (1979) J. Biol. Chem. 254, 3333–3340.
2. A. Sidhu (1990) J. Biol. Chem. 265, 10065–10072.
3. J. E. K. Hildreth (1982) Biochem. J., 207, 363–366.
4. A. K. Keenan, A. Gal, and A. Levitski (1982) Biochem. Biophys. Res. Commun. 105, 615–623.
5. J. M. Gonzalez-Ros, A. Paraschos, M. C. Farach, and M. Martinez-Carrion (1981) Biochim. Biophys. Acta. 643, 407–420.
6. T. Fujioka, F. Inoue, S. Sumita, and M. Kuriyama (1988) Biochem. Biophys. Res. Commun. 156, 54–60.
7. S. M. J. Dunn, R. A. Shelman, and M. W. Agey (1989) Biochemistry 28, 2551–2557.
8. K. W. Koch (1991) J. Biol. Chem. 266, 8634–8637.
9. T. N. Sato and J. H. Neal (1989) J. Neurochem. 52, 1114–1122.
10. A. K. Dutta-Roy and A. K. Sinha (1987) J. Biol. Chem. 262, 12685–12691.
11. H. Okamura, S. Raguet, A. Bell, J. Gagnon, and P. A. Kelly (1989) J. Biol. Chem. 264, 5904–5911.
12. A. P. Turkewitz, J. F. Amatruda, D. Borkani, S. C. Harris, and A. L. Schwartz (1987) J. Biol. Chem. 263, 8318–8325.
13. Y. F. Yamaguchi and J. T. Harmon (1988) Biochemistry 27, 3252–3260.
14. J. H. P. Skene and I. Virag (1989) J. Cell Biol. 108, 613–624.
15. R. Fukunaga, E. Ishizaka-Ikeda, and S. Nagata (1990) J. Biol. Chem. 265, 14008–14015.
16. E. A. Frey, M. E. Gosse, and T. E. Cote (1989) Eur. J. Pharmacol. 172, 347–356.
17. J. Mazella, J. Chabry, and J. P. Vincent (1989) J. Biol. Chem. 264, 5559–5563.
18. H. T. He, K. Johnson, K. Thermos, and T. Reisine (1989) Proc. Natl. Acad. Sci. USA 86, 1480–1484.
19. R. Munshi and J. Linden (1989) J. Biol. Chem. 264, 14853–14859.
20. D. J. Hryb, M. S. Khan, N. A. Romas, and W. Rosner (1989) J. Biol. Chem. 264, 5378–5383.
21. Y. H. Wong, C. D. Demoliou-Mason, and E. A. Barnard (1989) J. Neurochem. 52, 999–1009.
22. A. Mills, C. D. Demoliou-Mason, and E. A. Barnard (1988) J. Biol. Chem. 263, 13–16.
23. R. A. Cerione et al. (1983) Proc. Natl. Acad. Sci. USA 80, 4899–4903.
24. U. Kanngiesseer, P. Nalik, and O. Pongs (1988) Proc. Natl. Acad. Sci. USA 85, 2969–2973.
25. P. Banerjee, J. T. Buse, and G. Dawson (1990) Biochim. Biophys. Acta. 1044, 305–314.
26. P. Banerjee, J. B. Joo, J. T. Buse, and G. Dawson (1995) Chem. Phys. Lipids 77, 65–78.
27. J. Sambrook, E. F. Fritsch, and T. Maniatis (1989) Molecular Cloning, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring, NY, section 1.21.
28. P. Chomczynski and N. Sacchi (1987) Anal. Biochem. 162, 156–159.
29. L. Freysz et al. (1982) In Phospholipids in the Nervous System, Vol. 1 (Metabolism) (L. Horrocks et al., eds.), Raven Press, New York pp. 37–47.
30. R. N. Fontaine, R. A. Harris, and F. Schroeder (1980) J. Neurochem, 34, 269–277.
31. R. N. Fontaine, R. A. Harris, and F. Schroeder (1979) Life Sci. 24, 295–400.
32. M. J. Berridge (1984) Biochem. J. 220, 345–360.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
قسم بين الحرمين ينشر لافتات الحزن والعزاء في ذكرى فاجعة هدم قبور أئمة البقيع (عليهم السلام)
|
|
|