


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 8-5-2021
Date: 21-12-2015
Date: 11-6-2021
|
Biotin Repressor
All genes for the biosynthesis of biotin of Escherichia coli are grouped in a single operon located at 17 min on the chromosome, except for except for bioH, which is located at 75 min. The genes of the operon bioA,BFCD are transcribed divergently from a single regulatory region located between the bioA and the bioB genes. Transcription in both directions is corepressed by biotinyl-5′-adenylate and the biotin repressor. The following table shows the respective function of these genes:
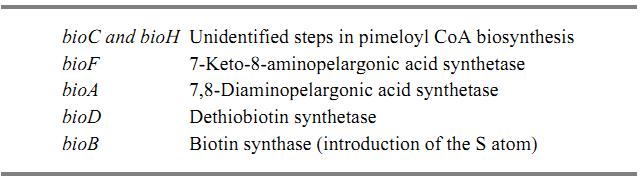
No precise information is available on the function of the bioC and bioH genes, other than that a mutation in either gene results in no excretion of a known intermediate in the pathway. Therefore the products of these genes have been assigned to some early steps before 7-keto-8-aminopelargonic acid synthesis. All biotin genes are coordinately repressed when biotin is added to the growth medium in excess of 1 ng/Ml.
The biotin repressor is a very interesting bifunctional protein of 321 amino acid residues, which acts at two different levels. In addition to its repressor function, it is endowed with acetyl CoA carboxylase biotin holoenzyme synthetase activity, which activates biotin to biotinyl-5′-adenylate and transfers the biotin to acceptor proteins. As soon as these proteins are totally biotinylated, biotinyl-5′-adenylate accumulates and serves as the corepressor of the biotin operon. This is a case of an enzyme synthesizing its own repressor, a unique property thus far among DNA-binding proteins. Mutations in the corresponding gene (birA) inactivate the repressor function partially or totally and also alter the enzymatic function. This repressor binds to a 40-bp symmetrical biotin operator site to prevent transcription of the biotin biosynthetic genes. The structure of the repressor is highly asymmetrical and consists of three domains. The N-terminal domain is mostly alpha-helical, contains a helix–turn–helix motif and is loosely connected to the remainder of the molecule. The central domain consists of a seven-stranded mixed beta-sheet, with a-helices covering one face. The other side of the sheet is largely exposed to the solvent and contains the enzyme's active site. The C-terminal domain comprises a six-stranded antiparallel b-sheet sandwich. The location of biotin is consistent with mutations affecting enzymatic activity.
Two molecules of the monomeric repressor bind cooperatively to one molecule of operator in the nanomolar concentration range. The data suggest that one molecule of repressor monomer binds to each of the two operator half sites and that they form a dimer only after they bind. Because the complex between repressor and DNA has not yet been crystallized, details of its structure remain an open question.



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
لحماية التراث الوطني.. العتبة العباسية تعلن عن ترميم أكثر من 200 وثيقة خلال عام 2024
|
|
|