


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 9-12-2015
Date: 6-11-2015
Date: 8-11-2015
|
Interactions between Cells of the Immune System
T Helper Cells (CD4+ T Cells) and T-B Cell Collaboration
Mature T cells expressing CD4 are called T helper (Th) cells, reflecting their role in co-operating with B cells. Foreign antigens, whose three-dimensional structures are recognized by B cells, also contain linear peptides. During the initial phase of the T helper cell response, these antigens are taken up by APCs, processed, and presented as peptides in association with MHC class II molecules—allowing their recognition by Th cells. Prior to our understanding of MHC restriction, B-cell epitopes were known as haptens, whilst those parts of the antigens which bore the T-cell epitope were known as carriers. 1n order for T cell activation to occur, antigen-transporting APCs must first reach the organized secondary lymphoid organs (Fig. 1), since proper contact between lymphocytes and APCs can only take place within these highly organized and compartmentalized organs. The cytokines IFNy, 1L-1,1L-2,1L-4, and 1L-12 play an important role in this process—as do various other factors.
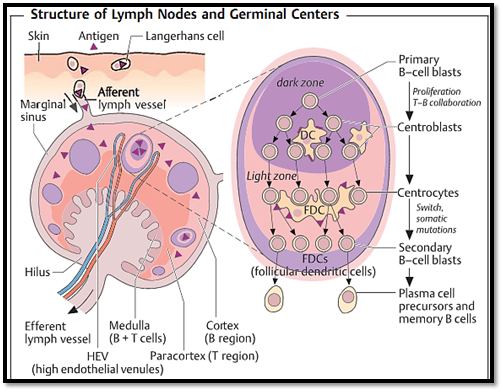
Fig. 1 Antigen carried by antigen-presenting cells (e.g., Langerhans cells in the skin which have taken up local antigens), or soluble antigens enter the marginal sinus of the lymph node through afferent lymphatic vessels. In the spleen, blood- borne antigens are taken up by specialized macrophages present in the marginal zone (marginal zone macrophages, MZM). Each lymph node has its own arterial and venous vascularization. Tand B cells migrate from blood vessels, through specialized venules with a high endothelium (HEV: high endothelial venules), into the paracortex which is largelycomprised ofT cells. Clusters of B cells (so-called primary follicles) are located in the cortex, where following antigen-stimulation, secondary follicles with germinal centers develop (right side). Active B-cell proliferation occurs at this site. Differentiation of B cells begins with the proliferation of the primary B- cell blasts within the dark zone and involves intensive interaction with antigen-presenting dendriticcells (DC). Antibody class switching and somatic mutation follows and takes place in the light zone, where FDC (follicular dendritic cells) stimulate B cells and store the antigen-antibody complexes that function to preserve antibody memory. Secondary B-cell blasts develop into either plasma cells or memory B cells. Lymphocytes can only leave the lymph nodes through efferent lymph vessels.
During the second phase (Fig. 2), activated T helper cells recognize the same MHC class II peptide complex, but on the surface of a B cell. Prior to this event, the B cell must have responded to the same antigen (by virtue of its Ig surface receptor recognizing a conformational antigenic epitope), then internalized the antigen, processed it, and finally presented parts of it in the form of linear peptides bound to MHC class II molecules on the cell surface for recognition by the T helper cell. The resulting B-T cell contact results in further interactions mediated by CD4, CD40, and CD28and sends a signal to the B cell which initiates the switch from IgM to IgG or other Ig classes. 1t also allows induction of a process of somatic mutation, and probably enhances the survival of the B cell in the form of a memory B cell.

Fig. 2 For the sake of simplicity, the principles illustrated here are based on an antigen (1) which only contains a single B epitope and a single T epitope. As an example, the structural B epitope (blue) is presenton the surface of the antigen; whilst the linear T epitope (red) is hidden inside it. An antigen-presenting cell (APC), or macrophage, takes up the antigen and breaks it down in a nonspecific manner. The T-cell epitope is thus released and loaded onto MHC class II molecules which are presented on the cell surface (2). AT helper cell specifically recognizes the Tepitope presented by the MHC class II molecule. This recognition process activates the APC (3a) (or the macrophages). T cells, APC, and macrophages all produce cytokines (Fig. 3), which then act onTcells, B cells, and APCs (causing up-regulation of CD40, B7)(3). This in turn stimulates the T cells to proliferate, and encourages the secretion of additional signaling substances (IL-2, IFNy, IL-4, etc.). A B cell whose surface Ig has recognized and bound a B epitope present on the intact antigen, will present the antigenic T cell epitope complexed to MHC class II on its cell surface, in a manner similar to that described for the APC (4). This enables direct interaction between the T helper cell and the specific B cell, resulting in induction of proliferation, differentiation, and B-cell class switching from IgM to other Ig classes. The B cell finally develops into an antibody-producing plasma cell. The antibody-binding site of the produced antibody thus fits the B epitope on the intact antigen. The induction of cytotoxic effector cells by peptides presented on MHC class I molecules (violet) is indicated in the lower part of the diagram (5). The cytotoxic Tcell precursors do not usually receive contact-mediated T help, but are rather supported by secreted cytokines (mainly IL-2) (6). (Again, in the interest of simplicity, the CD3 and CD4 complexes and cytokines are not shown in detail)
Subpopulations of T Helper Cells
Soluble signaling substances, cytokines (interleukins), released from T helper cells can also provide an inductive stimulus for B cells. Two subpopulations of T helper cells can be differentiated based on the patterns of cytokines produced (Fig. 3). Infections in general, but especially those by intracellular parasites, induce cytokine production by natural killer (NK) cells in addition to a strong T helper 1 (TH1) response. The response by these cells is characterized by early gamma interferon (IFNy) production, increased levels of phagocyte activity, elimination of the antigen by IFNy-activated macrophages, production of 1gG2a and other complement-binding (opsonizing) antibodies (see the complement system, pp. 86ff.), and induction of cytotoxic T-cell responses. IL-12 functions as the most important promoter of TH1 cell function and additionally acts as an inhibitor of TH2 cells.
1n contrast, worm infections or other parasitic diseases induce the early production of IL-4, and result in the development of a TH2 response. TH2 cells, in turn, recruit eosinophils and induce production of IgGI and IgE antibodies. Persons suffering from allergies and atopic conditions show a pathologically excessive TH2 response potential. 1L-4 not only promotes the TH2 response but also inhibits TH1 cells.
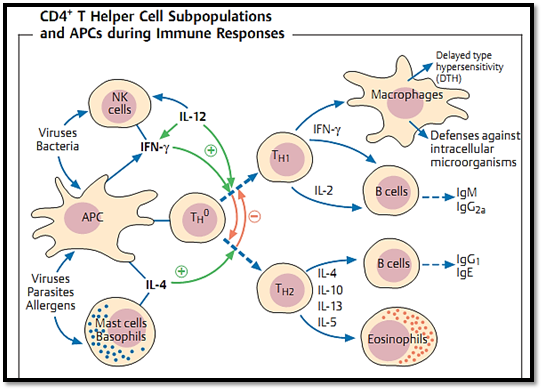
Fig. 3 TH1 and TH2 cells are derived from a TH0 cell, and undergo differentiation in the presence of help derived from cytokines, DC, macrophages, and other cell types. TH1 cells are activated byIL-12and IFNy and inhibited by IL-4; whilst for TH2 cells the reverse is true. Viruses and bacteria (particularly intracellular bacteria) can induce a TH1 response by activating natural killer cells. In contrast, allergens and parasites induce a TH2 response via the release of IL-4. However, the strong in- vitro differentiation of CD4+ Tcells intoTH1-TH2 subsets is likely to be less sharply defined in vivo.
Cytotoxic T Cells (CD8+ T Cells)
Mature CD8+ T cells perform the biologically important function of lysing target cells. Target cell recognition involves the association of MHC class I structures with peptides normally derived from endogenous sources, i.e., originating in the cells themselves or synthesized within them by intracellular parasites. Induction of cytotoxic CD8+ T cell response often does not require helper cells—or only requires these cells indirectly. However, should the antigen stimulus and the accompanying inflammation be of a low-level nature, the quantity of cytokines secreted by the cytotoxic T cells themselves may not suffice, in which case the induction of a CD8+ T cell response will be reduced unless additional cytokines are provided by helper T cells. The cytotoxic activity of CD8+ T cells is mediated via contact and perforin release (perforin renders the membrane of the target cell permeable resulting in cellular death). CD8+ T cells also function in interleukin release (mainly of IFNy) by which they mediate non-cytotoxic effector functions (Fig. 4). The role of perforin in contact-dependent direct cytolysis by natural killer (NK) cells and cytotoxic T cells has been investigated in gene knockout mice. In these animals the perforin gene has been switched off by means of homologous recombination, and as a result they can no longer produce perforin. Per-forin-dependent cytolysis is important for the control of noncytopathicviruses, tumors, and transformed cells, but also plays a large role in the control of highly virulent viruses that produce syncytia (e.g., the smallpox virus). Release of noncytolytic effector molecules by CD8+ cells, mostly IFNy, plays a major role in control of cytopathic viruses and intracellular bacteria. Cytolytic effector mechanisms may also contribute to release of intracellular micro-organisms and parasites (e.g., tuberculosis) from cells that only express MHC class I.
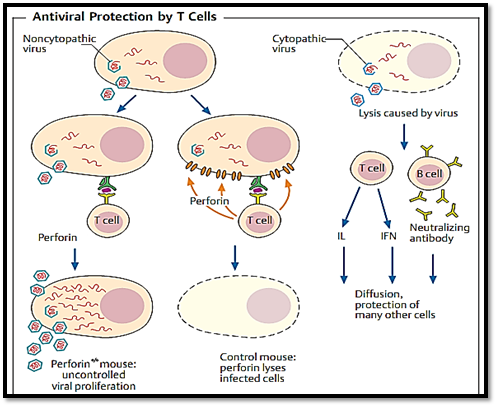
Fig. 4 Certain viruses destroy the infected host cells (right), others do not (left). Cytotoxic T cells can destroy freshly infected cells by direct contact (with the help of perforin), thus inhibiting viral replication (middle). Whether the result of this lysis is clinically desirable depends on the balance between protection from viral proliferation, and the damage caused by immunologically mediated cell destruction. In perforin knockout mice (perforino/o), T cells are unable to produce perforin and therefore do not destroy the infected host cells. Replication of non-cytopathic viruses thus continues unabated in these mice. Soluble anti-viral interleukins (especially IFNy and TNFa), and neutralizing antibodies, combat cytopathic viruses (which replicate comparatively rapidly) more efficiently than do cytolytic T cells; this is because interleukin and antibody molecules can readily diffuse through tissues and reach a greater number of cells, more rapidly, than can killer T cells.
Cytokines (Interleukins) and Adhesion
Cytokines are bioactive hormones, normally glycoproteins, which exercise a wide variety of biological effects on those cells which express the appropriate receptors (Table 1). Cytokines are designated by their cellular origin such that monokines include those interleukins produced by macrophages/ monocytes, whilst lymphokines include those interleukins produced by lymphocytes. The term interleukins is used for cytokines which mostly influence cellular interactions. All cytokines are cyto-regulatory proteins with molecular weights under 60 kDa (in most cases under 25 kDa). They are produced locally, have very short half-lives (a matter of seconds to minutes), and are effective at picomolar concentrations. The effects of cytokines may be paracrine (acting on cells near the production locus), or autocrine (the same cell both produces, and reacts to, the cytokine). By way of interaction with highly specific cell surface receptors, cytokines can induce cell-specific or more general effects (including mediator release, expression of differentiation molecules and regulation of cell surface molecule expression). The functions of cytokines are usually pleiotropic, in that they display a number of effects of the same, or of a different, nature on one or more cell types. Below is a summary of cytokine functions:
-Promotion of inflammation: IL-1, IL-6, TNFα, chemokines (e.g., IL-8).
-Inhibition of inflammation: IL-10, TGFβ.
-Promotion of hematopoiesis: GM-CSF, IL-3, G-CSF, M-CSF, IL-5, IL-7
Activating B cells: CD40L, IL-6, IL-3, IL-4.-
-Activating T cells: IL-2, IL-4, IL-10, IL-13, IL-15.
Anti-infectious: IFNα, IFNβ, IFNγ, TNFα.-
-Anti-proliferative: IFNα, IFNβ, TNFα, TGFβ.
Table 1 The Most Important Immunological Cytokines and Costimulators plus Their Receptors and Functions
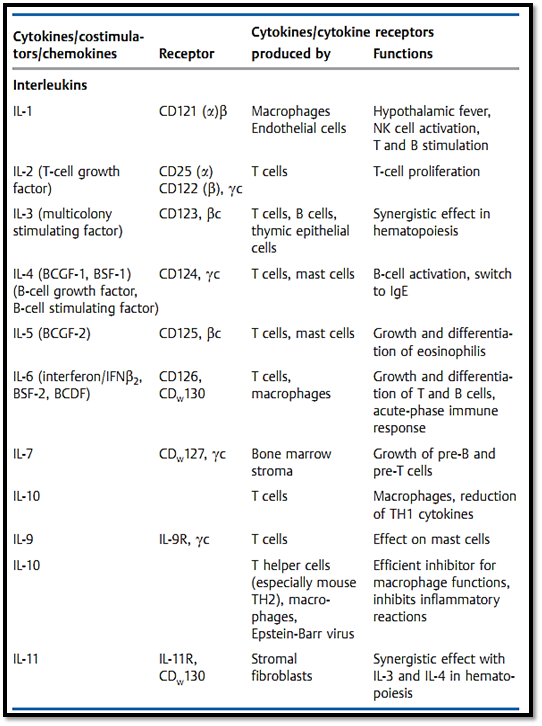
Table 1 Continued: The Most Important Immunological Cytokines .
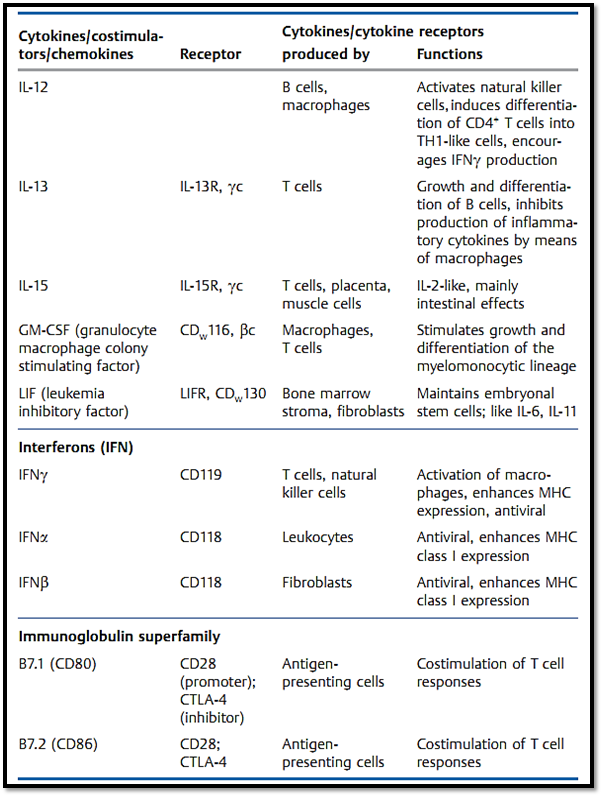
Table 1 Continued: The Most Impor tant Immunological Cytokines . .
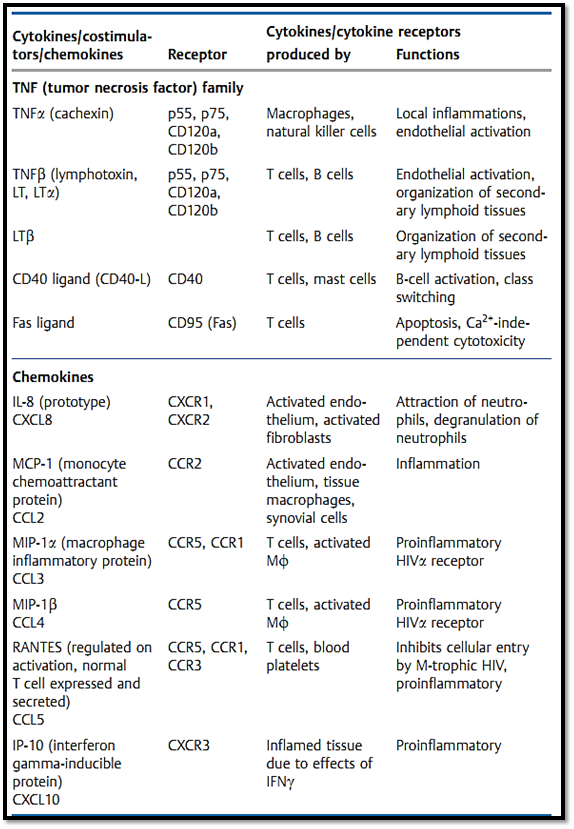
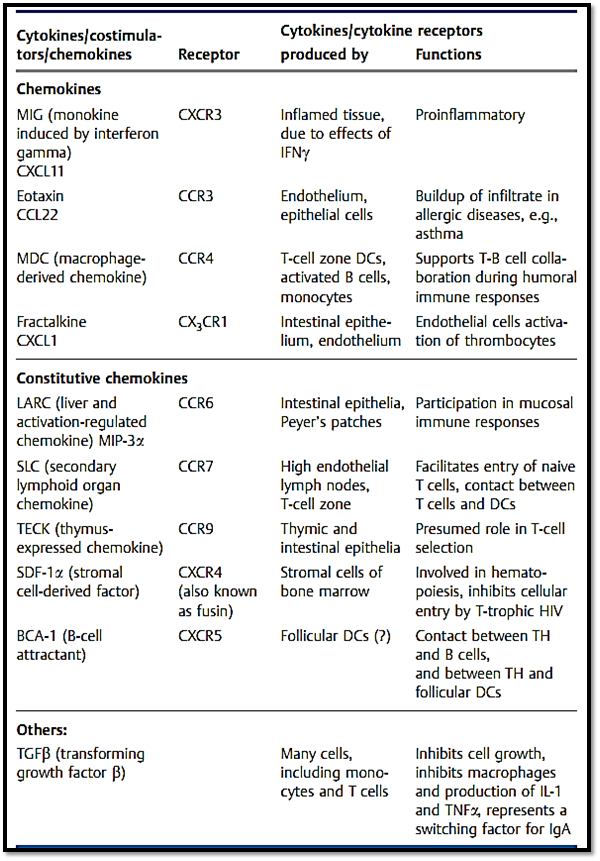
Table 1 Continued: The Most Impor tant Immunological Cytokines . . .
Cell adhesion molecules often play an essential role in cell-to-cell interactions. Two lymphohematopoietic cells can only establish contact if one of them expresses surface molecules that interact with ligands expressed on the surface of the other cell. As for APC and T cell interactions, the result of such contact may be that a signal capable of inducing differentiation and functional changes will be induced. Adhesion proteins are usually comprised of several chains which can induce different effects when present in various combinations. Interaction of several cascades is often required for the final differentiation of a cell. Cell adhesion molecules normally form part of the Ig superfamily (e.g., ICAM, VCAM, CD2), integrin family (lymphocyte function antigen, LFA-1), selectin family, cadherin family, or various other families. Selectins and integrins also play an important role in interactions between leukocytes and the vascular wall, and thus mediate the migration of leukocytes from the bloodstream into inflamed tissues, or the entry of recirculating lymphocytes into the lymph node parenchyma through high endothelial venules (HEV).
Chemokines (chemoattractant cytokines) comprise a family of over 30 small (8-12 kDa) secreted proteins. These contribute to the recruitment of “inflammatory cells” (e.g., monocytes) into inflamed tissues, and influence the recirculation of all classes of leukocytes (Table 1). Some chemokines result in the activation of their target cell in addition to exerting chemotatic properties. Chemokines can be classified into three families based on their N terminus structure: CC chemokines feature two contiguous cysteine residues at the terminus; CXC chemokines have an amino acid between the two residues; and CX3Cand C chemokines thus far comprise only one member each (fractalkine and lymphotactin, respectively). Although the N terminus carries bioactive determinants, using a chemokines amino acid sequence to predict its biological function is not reliable. The chemokine system forms a redundant network, or in other words, a single chemokine can often act upon a number of receptors, and the same receptor may recognize a number of different chemokines. Many of the chemokines also overlap in terms of biological function.
Chemokines can be grouped in two functional classes: inflammatory che- mokines which are secreted by inflamed or infected tissues as mediators of the nonspecific immune response; and constitutive chemokines which are produced in primary or secondary lymphoid organs. Together with endothelial adhesion molecules, inflammatory chemokines determine the cellular composition of the immigrating infiltrate. In contrast, the function of constitutive chemokines is to direct lymphocytes to precise locations within lymphoid compartments. Thus, chemokines play a major role in the establishment of inflammatory and lymphoid microenvironments. Chemokine receptors are G protein-coupled membrane receptors with seven transmembrane sequences. In keeping with the above nomenclature, they are designated as CCR, CXCR, or CX3CR plus consecutive numbering. Some viruses, for instance chemokines, and may offer an advantage to the virus. The Duffy antigen receptor for chemokines, DARC, is expressed on endothelial cells and is capable of a high-affinity binding interaction with various chemokine types. Since this receptor has no downstream signaling cascade, it is assumed to function in the presentation of chemokines to leukocytes as they flow past. DARC also functions as a receptor for Plasmodium vivax. CCR5 and CXCR4 are co-receptors for HIV infection of CD4+ T cells.
Antibody-Dependent Cellular Immunity and Natural Killer Cells
Lymphocytes can nonspecifically bind IgG antibodies by means of Fc receptors, then specifically attack targets cells (e.g., infected or transformed cells) using the bound antibody. This phenomenon, known as antibody-dependent cellular cytotoxicity (ADCC), has been demonstrated in vitro—however its in-vivo function remains unclear. Natural killer (NK) cells also play a role in ADCC. The genesis of NK cells appears to be mainly thymus-independent. These cells can produce IFNγ very early following activation and do not require a specific receptor. These cells are therefore early contributors to the IFNγ-oriented TH1 immune response. NK cells can respond to cells that do not express MHC class I molecules, and are inactivated by contact with MHC molecules. This recognition process functions via special receptors that are not expressed in a clonal manner. NK cells probably play an important role in the early defensive stages of infectious diseases, although the exact nature of their role remains to be clarified (virus-induced IFNα and IFNβ promote NK activation). NK cells also appear to contribute to rejection reactions, particularly the rejection of stem cells.
Humoral, Antibody-Dependent Effector Mechanisms
The objectives of the immune response include: the inactivation (neutralization) and removal of foreign substances, microorganisms, and viruses; the rejection of exogenous cells; and the prevention of proliferation of pathologically altered cells (tumors). The systems and mechanisms involved in these effector functions are largely non-specific. Specific immune recognition by B and T cells directs these effector mechanisms to specific targets. For instance, immunoglobulins opsonize microbes (e.g., pneumococci) which are equipped with polysaccharide capsules enabling them to resist phagocyte digestion. Opsonization involves the coating of such microbes with Fc-ex- pressing antibodies which facilitates their phagocytosis by granulocytes. Many cells, particularly phagocytes (and interestingly enough also some bacteria like staphylococci), bear surface Fc receptors that interact with different Ig classes and subclasses. Mast cells and basophils bear IgE molecules, and undergo a process of degranulation following interaction with allergens against which the IgE molecules are directed. This induces the release of pharmacologically active biogenic amines (e.g., histamine). In turn, these amines represent the causative agent for physiological and clinical symptoms observed during allergic reactions.
References
Zinkernagel, R. M. (2005). Medical Microbiology. © Thieme.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|