


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 8-12-2020
Date: 6-11-2015
Date: 8-11-2015
|
Immunological Memory
Immunological memory is usually defined by an earlier and better immune response, mediated by increased frequencies of specific B or T cells as determined by in vitro or adoptive transfer experiments. B-cell immunological memory is more completely described as the ability to mediate protective immunity by means of increased antibody concentrations. Higher frequencies of specific B and T lymphocytes alone, appears to only provide limited or no protection. Instead, immunological protection requires antigen-dependent activation of B and Tcells, which then produce antibodies continuously or can rapidly mediate effector T functions and can rapidly migrate into peripheral tissues to control virus infections.
Usually the second time a host encounters the same antigen its immune response is both accelerated and augmented. This secondary immune response is certainly different from the primary response, however, it is still a matter of debate as to whether these parameters alone correlate with immune protection. It is not yet clear whether the difference between a primary and secondary immune response results solely from the increased numbers of antigen-specific B and T cells and their acquisition of “memory qualities”, or whether immune protection is simply due to continuous antigen-induced activation (Table 1).
Table 1 Characteristics of T- and B-Cell Memory
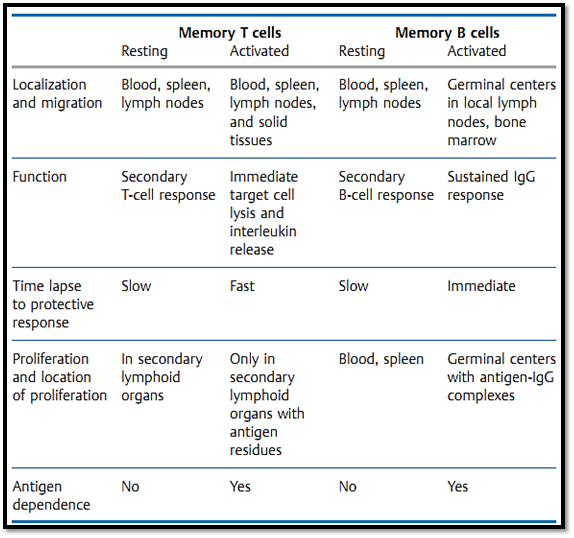
There is no surface marker which can unequivocally differentiate between memory T and B cells and “naive” (never before activated) cells. Instead, immunological memory is normally taken to correlate with an increased number of specific precursor T and B cells. Following an initial immunization with antigen, this increased precursor frequency of specific cells is thought to be maintained by an antigen-independent process. Yet the precursor cells can only be activated (or re-activated) by antigen, and only activated Tcells can provide immediate protection against re-infection outside the lymphoid organs, e.g., in the solid peripheral organs. Similarly, only antigen activated B cells can mature to become plasma cells which maintain the increased blood antibody titers responsible for mediating protection. This indicates that residual antigen must be present to maintain protective immunological memory. As a general rule, the level of protective immunity mediated by the existence of memory T and B cells per se is minimal. Highly effective immunity and resistance to re-infection are instead provided by migratory T cells which have been recently activated (or re-activated) by antigen, and by antibody- secreting B cells. B-cell and antibody memory is maintained by re-encounters with antigen, or by antigen-IgG complexes which by virtue of their Fc portions or by binding to C3b are captured by-, and maintained for long periods on-, follicular dendritic cells present in germinal centers. Memory T cells, and in some cases B cells, can be re-stimulated and maintained in an active state by: persistent infections (e.g., tuberculosis, hepatitis B, HIV); antigen deposits in adjuvants; periodic antigen re-exposure; peptide-loaded MHC molecules with long half-lives; or possibly (but rarely) by cross-reactive antigens. Thus, secondarily activated (protective) memory T and B cells cannot easily be distinguished from primarily activated T and B cells. The antigen-dependent nature of immunological protection indeed questions the relevance of a specialized “memory quality” of B and T cells.
B-Cell Memory
It is important to differentiate between the characteristics of memory T and B cells as detected in vitro, and the salient in-vivo attributes of improved immune defenses. Following a primary immune response, increased numbers of memory B cells can of course be detected using in vitro assays or by murine experiments involving the transfer of cells into naive recipients. However, these increased B cell frequencies do not necessarily ensure immune protection against, for instance, viral re-infection. Such protection requires the existence of an increased titer of protective antibodies within the host.
Why is Immunological Memory Necessary?
A host which does not survive an initial infection obviously does not require further immunological memory. On the other hand, survival of the initial infection proves that the host’s immune system can control or defeat the infection, once again apparently negating the need for immunological memory. Even assuming that better immune defenses provide a clear evolutionary advantage, especially during pregnancy, the idea of immunological memory must be understood as protection within a developmental framework:
1- 1-Due to MHC restriction of T-cell recognition, it is not possible fora mother to pass on T-cell immunological experience to her progeny as the histoincompatibility reaction would induce mutual cellular rejection. For the same reason, a child’s T cells apparently cannot mature until relatively late in its development (usually around the time of birth). This explains why newborns are almost entirely lacking in active immune defenses (Fig. 1). Newborn mice require about three to four weeks (humans three to nine months) before the T-cell immune response and the process of T-B cell collaboration which results in the generation of antibody responses become fully functional. During this period passive immune protection is essential. This type of protection is mediated by the transfer of protective, largely IgG, antibodies from mother to child through the placenta during pregnancy, and to some extent within the mother’s milk. An example of this is provided by cattle where the acquisition of colostral milk by the calf is essential to its survival. Calves can only access protective IgG through the colostral milk delivered during the first 24 hours after birth (fetal calf serum contains no Ig). During the first 18 hours post partum, the calf’s intestine expresses Fc receptors which allow the uptake of undigested antibodies from the mothers milk into the bloodstream. How can comprehensive, transferable, antibody-mediated protection be ensured under these conditions? During a three-week murine or 270-day human pregnancy, mothers do not normally undergo all of the major types of infection (indeed infection can be potentially life-threatening for both the embryo/fetus and the mother), and so the array of antibodies required for comprehensive protection cannot be accumulated during this period alone. Instead, an accumulation of the immunological protective antibody levels representing the immunological life experience of infections in the mother’s serum is necessary. The female sex hormones also encourage Ig synthesis, correlating with women’s higher risk level (about fivefold) for developing autoantibody diseases (e.g., lupus), and for autoimmune diseases in general.
2-Reproduction requires a relatively good level of health and a good nutritional status of the mother. However, it also requires an effective immune defense status within the population (herd), including males, since all would otherwise be threatened by repeated and severe infections. The increased frequency of specific precursor B and T cells improves immune defenses against such infections. However, this relative protection is in clear contrast to the absolute protection an immunoincompetent newborn requires to survive.
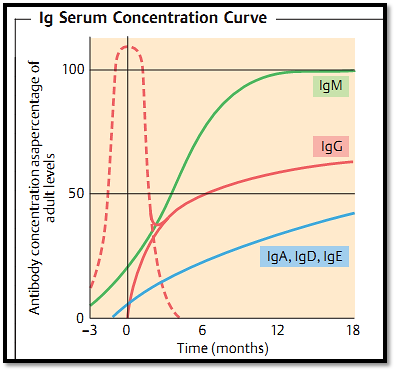
Fig. 1 Synthesis of significant amounts of immunoglobulins only begins during the perinatal period (uninterrupted lines). IgG from the mother is therefore the child’s main means of protective immunity before the age of three to six months (dotted line). Infections encountered during this early period are attenuated by maternal antibodies, rendering such infections vaccine-like.
T-Cell Memory
As with B cells and antibodies, enhanced defenses against intracellular pathogens (especially viruses and intracellular bacteria) does not solely depend on increased numbers of specific T cells, but rather is determined by the activation status of T cells. Here again it must be emphasized that protective immunological memory against most bacteria, bacterial toxins, and viruses, is mediated by antibodies! Memory T cells are nonetheless important in the control of intracellular bacterial infections (e.g., tuberculosis [TB], leprosy), as well as persistent noncytopathic viruses such as hepatitis B and HIV. It has been demonstrated, at least in mouse models, that a higher number of T cells alone is often insufficient for the protection of the host against the immunopathological consequences of a defensive CD8+ T-cell response. Yet such T cell responses must be activated in order to provide immunity. In the case of tuberculosis, sustained activation of a controlled T-cell response by minimal infection foci was postulated, and confirmed, in the 1960s as constituting infection immunity—i.e. the lifelong, and usually effective, immune control of the disease by an ongoing localized low-level of infection. A similar situation is observed for cell-mediated immune responses against leprosy, salmonellae, and numerous parasitic diseases (often together with antibodies). The existence of infection-immunity explains why apparently controlled, minimal, infections tend to flare up when the immune system is compromised by cytostatic drugs, age, or HIV infection. Delayed type(dermal) hypersensitivity (DTH, see below and p. 114f.) can be applied diagnostically to determine infection immunity (for example against tuberculosis and leprosy), since the existence of continued infection continuously activates those T cells required for both pathogen control and DTH reactions.
References
Zinkernagel, R. M. (2005). Medical Microbiology.



|
|
|
|
مخاطر عدم علاج ارتفاع ضغط الدم
|
|
|
|
|
|
|
اختراق جديد في علاج سرطان البروستات العدواني
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|