


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 6-11-2015
Date: 6-11-2015
Date: 8-11-2015
|
The B-Cell System
B lymphocytes produce antibodies in two forms; a membrane-bound form and a secreted form. Membrane-bound antibody forms the B-cell antigen receptor. Following antigen stimulation, B lymphocytes differentiate into plasma cells, which secrete antibodies exhibiting the same antigen specificity as the B-cell receptor. This system is characterized as humoral immunity, due to this release of receptors into the “humoral” system which constitutes vascular contents and mucous environments. The humoral system also contains non-specific defense mechanisms, including the complement system. In chemical terms, B-cell receptors are globulins (Ig or immunoglobulins). These immunoglobulins comprise a number of classes and subclasses, as well as numerous different specificities, but share a common structure (Fig. 1a).
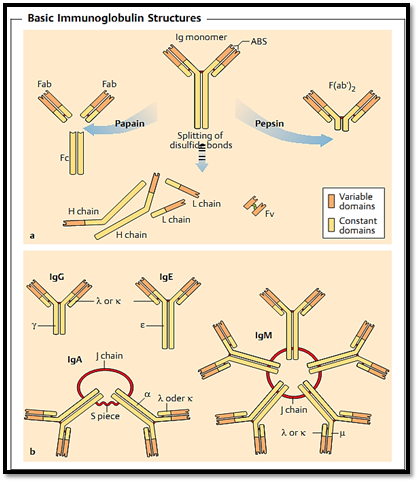
Fig. 1 a Immunoglobulin monomers. The upper half of the figure shows the intact monomer consisting of two L and two H chains. The positions of the disulfide bonds, the variable N-terminal domains, and the antigen-binding site (ABS) are indicated. The lower half of the figure shows the monomers of the individual polypeptide chains as seen following exposure to reducing conditions (which break the disulfide bonds) and denaturing conditions; note that the ABS is lost. Papain digestion produces two monovalent Fab fragments, and one Fc fragment. Following pepsin digestion (right), the Fc portion is fragmented, but the Fab fragments remain held together by disulfide bonds. The F(ab’)2 arm is bivalent (with two identical ABS). Fv fragments comprise a single-chain ABS formed by recombinant technology. These consist of the variable domains of the H and L chains, joined covalently by a synthetic linker peptide.
b Classes of immunoglobulins. IgM, IgD, IgG, IgA, and IgE are differentiated by their respective heavy chains (µ, δ, γ, α, ε). IgA (a chain) forms dimers held together by the J (joining) chain; the secretory (S) piece facilitates transport of secretory IgA across epithelial cells, and impairs its enzymatic lysis within secretions. IgM (i chain) forms pentamers with 10 identical ABS; the IgM monomers are held together by J chains. The light chains (k and k) are found in all classes of immunoglobulins.
Immunoglobulin Structure
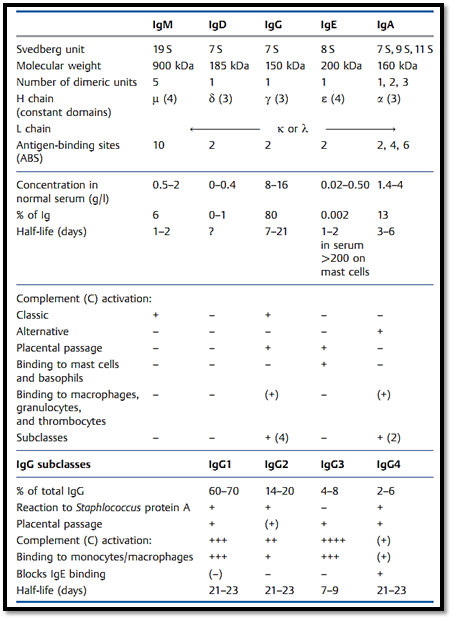
All immunoglobulin monomers have the same basic configuration, in that they consist of two identical light chains (L) and two identical heavy chains (H). The light chains appear as two forms; lambda (λ) or kappa (k). There are five main heavy chain variants; µ,δ, γ, α, and ε. The five corresponding immunoglobulin classes are designated as IgM, IgD, IgG, IgA, or IgE, depending on which type of heavy chain they use (Fig. 1b). A special characteristic of the immunoglobulin classes IgA and IgM is that these comprise a basic monomeric structure that can be doubled or quintupled (i.e., these can exist in a dimeric or pentameric form). Table 1 shows the composition, molecular weights and serum concentrations of the various immunoglobulin classes .
Table 1 Characteristics of the Various Immunoglobulin Classes
Immunoglobulins contain numerous domains, as illustrated by the structure of IgG. In monomeric IgG each domain consists of a protein segment which is approximately 110 amino acids in length. Both light chains possess two such domains, and each heavy chain possesses four or five domains. The domain structure was first revealed by comparison of the amino acid sequence derived from many different immunoglobulins belonging to the same class. In this way a high level of sequence variability was revealed to be contained within the N-terminal domain (variable domain, V), whilst such variability was comparably absent within the other domains (constant domains, C). Each light chain consists of one variable domain (VL) and one constant domain (CL). In contrast, the heavy chains are roughly 440-550 amino acids in length, and consist of four to five domains. Again, the heavy chain variable region is made up of one domain (VH), whereas the constant region consists either of three domains (y, a, § chains), or four domains (i, e chains) (CH1, CH2, CH3, and CH4). Disulfide bonds link the light chains to the heavy chains and the heavy chains to one another. An additional disulfide bond is found within each domain.
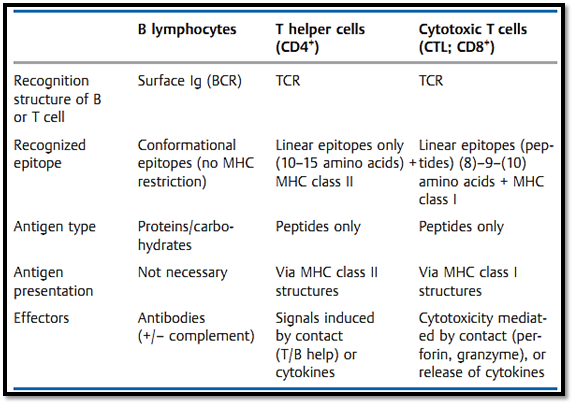
The three-dimensional form of the molecule forms a letter Y. The two short arms of this 'Y' consist of four domains each (VL, CL, VH, and CH1), and this structure contains the antigen-binding fragments—hence its designation as Fab (fragment antigen binding). The schematic presented in Fig. 2.3 is somewhat misleading, since the two variable domains of the light and heavy chains are in reality intertwined. The binding site—a decisive structure for an epitope reaction—is formed by the combination of variable domains from both chains. Since the two light chains, and the two heavy chains, contain identical amino acid sequences (this includes the variable domains), each immunoglobulin monomer has two identical antigen-binding sites (ABS), and these form the ends of the two short arms of the 'Y'. An area within the antibody consisting of12-15 amino acids contacts the peptide region contained within the antigen and consisting of approximately 5-800 A2 (Table 2). The trunk of the 'Y' is called the Fc fragment (named, “fraction crystallizable” since it crystallizes readily) and is made up of the constant domains of the heavy chains (CH2 and CH3, and sometimes CH4).
Table 2 Antigen Recognition by B and T Cells
Diversity within the Variable Domains of the Immunoglobulins
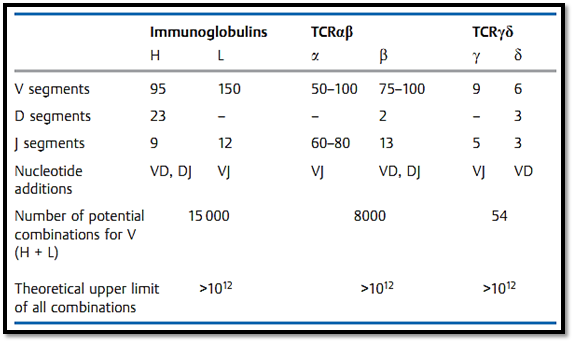
The specificity of an antibody is determined by the amino acid sequence of the variable domains of the H and L chains, and this sequence is unique for each corresponding cell clone. How has nature gone about the task of producing the needed diversity of specific amino acid sequences within a biochemically economic framework? The genetic variety contained within the B-cell population is ensured by a process of continuous diversification of the genetically identical B-cell precursors. The three gene segments (variable, diversity, joining) which encode the variable domain (the VDJ region for the H chain, and the VJ region for the L chain) are capable of undergoing a process called recombination. Each of these genetic segments are found as a number of variants (Fig. 2, Table 3). B-cell maturation involves a process of genetic re-
Table 3 Organization of the Genetic Regions for the Human Immunoglobulins and T-Cell Receptor s (TCR)
combination resulting in a rearrangement of these segments, such that one VH, one DH, and one JH segment become combined. Thus the germ line does not contain one gene governing the variable domain, but rather gene segments which each encode fragments of the necessary information. Mature B cells contain a functional gene which, as a result of the recombination process, is comprised of one VHDHJH segment. The diversity of T-cell receptors is generated in a similar manner.
Fig. 2 explains the process of genetic recombination using examples of an immunoglobulin H chain and T-cell receptor a chain.
The major factors governing immunoglobulin diversity include:
Multiple V gene segments encoded in the germ lines-
The process of VJ, and VDJ, genetic recombination-
Combination of light and heavy chain protein structures-
-Random errors occurring during the recombination process, and inclusion of additional nucleotides
Somatic point mutations-
In theory, the potential number of unique immunoglobulin structures that could be generated by a combination of these processes exceeds 1012, however, the biologically viable and functional range of immunoglobulin specificities is likely to number closer to 104.
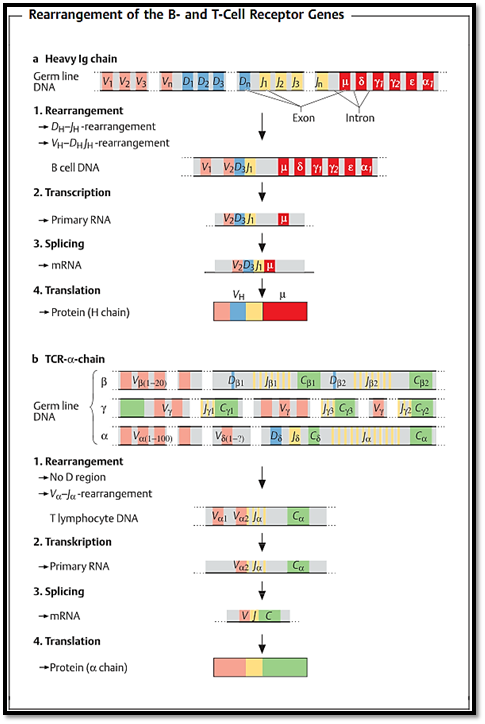
Fig. 2 a Heavy chain of human IgG. The designations for the gene segments in the variable part of the H chain are V (variable), D (diversity), and J (joining). The segments designated as µ, δ, γ, α, and ε code for the constant region and determine the immunoglobulin class. The V segment occurs in several hundred versions, the D segment in over a dozen, and the J segment in several forms. V, D, and J segments combine randomly to form a sequence (VDJ) which codes for the variable part of the H chain. This rearranged DNA is then transcribed, creating the primary RNA transcript. The non-coding intervening sequences (introns) are then spliced out, and the resulting mRNA is translated into the protein product. b a chain of mouse T-cell receptor. Various different V, D, and J gene segments (for b and 5), V and J gene segments (for a and y) are available for the T-cell receptor chains. The DNA loci for the 5 chain genes are located between those for the a chain.
The Different Classes of Immunoglobulins
Class switching. The process of genetic recombination results in the generation of a functional VDJ gene located on the chromosome upstream of those regions encoding the H chain segments Cµ, Cδ, Cγ, Cα, and Cε, in consecutive order. Thus all immunoglobulin production begins with the synthesis of IgM and IgD (resulting from transcription of the VDJ and the Cµ or Cδ gene segments). This occurs without prior antigen stimulus and is transitional in nature. Antigen stimulation results in a second gene rearrangement—during which the VDJ gene is relocated to the vicinity of Cγ, Cα, or Cε by a process of recombination involving deletion of the intervening regions. Following this event, the B cell no longer produces H chains of the IgM or IgD classes, but is instead committed to the production of IgG, IgA, or IgE—thus allowing secretion of the entire range of immunoglobulin types (Table 2.2). This process is known as class switching, and results in a change of the Ig class of an antibody whilst allowing its antigen specificity to be retained.
Variability types. The use of different heavy or light chain constant regions results in new immunoglobulin classes known as isotypes. Individual Ig classes can also differ, with such genetically determined variations in the constant elements of the immunoglobulins (which are transmitted according to the Mendelian laws) are known as allotypes. Variation within the variable region results in the formation of determinants, known as idiotypes. The idiotype determines an immunoglobulins antigenic specificity, and is unique for each individual B-cell clone.
Functions. Each different class of antibody has a specific set of functions. IgM and IgD act as B-cell receptors in their earlier transmembrane forms, although the function of IgD is not entirely clear. The first antibodies produced in the primary immune response are IgM pentamers, the action of which is directed largely against micro-organisms. IgM pentamers are incapable of crossing the placental barrier. The immunoglobulin class which is most abundant in the serum is IgG, with particularly high titers of this isotype being found following secondary stimulation. IgG antibodies pass through the placenta and so provide the newborn with a passive form of protection against those pathogens for which the mother exhibits immunity. In certain rare circumstances such antibodies may also harm the child, for instance when they are directed against epitopes expressed by the child's own tissues which the mother has reacted against immunologically (the most important clinical example of this is rhesus factor incompatibility). High concentrations of IgA antibodies are found in the intestinal tract and contents, saliva, bronchial and nasal secretions, and milk—where they are strategically positioned to intercept infectious pathogens (particularly commensals) (Fig. 3). IgE antibodies bind to high-affinity Fce receptors present on basophilic granulocytes and mast cells. Cross-linking of mast cell bound IgE antibodies by antigen results in cellular degranulation and causes the release of highly active biogenic amines (histamine, kinines). IgE antibodies are produced in large quantities following parasitic infestations of the intestine, lung or skin, and play a significant role in the local immune response raised against these pathogens.
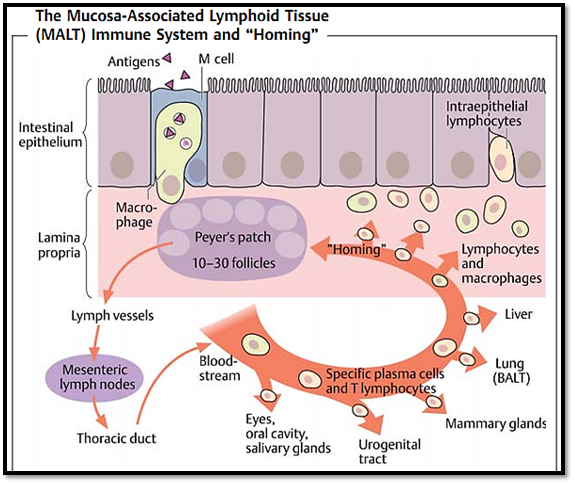
Fig. 3 Specialized APCs (M cells in the intestinal wall or pulmonary macrophages in the lung) take up antigens in mucosa and present them in the Peyer’s patches or local lymph nodes. This probably enhances T cell-dependent activation of IgA-producing B cells, which are preferentially recruited to the mucosal regions (“homing”) via local adhesion molecules and antigen depots, resulting in a type of geographic specificity within the immune response.
References
Zinkernagel, R. M. (2005). Medical Microbiology. © Thieme.



|
|
|
|
صنع الذكريات والتفكير يدمر الدماغ.. دراسة تشرح السبب
|
|
|
|
|
|
|
الصين.. عودة كاسحتي الجليد إلى شنغهاي بعد انتهاء بعثة استكشافية إلى القطب الجنوبي
|
|
|
|
|
|
جامعة الكفيل تكرم الفائزين بأبحاث طلبة كلية الصيدلة وطب الأسنان
|
|
|
|
مشروع التكليف الشرعي بنسخته السادسة الورود الفاطمية... أضخم حفل لفتيات كربلاء
|
|
|
|
ضمن جناح جمعيّة العميد العلميّة والفكريّة المجمع العلمي يعرض إصداراته في معرض تونس الدولي للكتاب
|
|
|
|
جامعة الكفيل تعقد مؤتمرها الطلابي العلمي الرابع
|