


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 26-4-2016
Date: 7-5-2021
Date: 24-3-2021
|
The lac Repressor Is a Tetramer Made of Two Dimers
KEY CONCEPTS
- A single repressor subunit can be divided into the Nterminal DNA-binding domain, a hinge, and the core of the protein.
- The DNA-binding domain contains two short α-helical regions that bind the major groove of DNA.
- The inducer-binding site and the regions responsible for multimerization are located in the core.
- The monomers form a dimer by making contacts between core subdomains 1 and 2.
- The dimers form a tetramer by interactions between the tetramerization helices.
- Different types of mutations occur in different domains of the repressor protein.
The repressor protein has several domains, as shown in the crystal structure illustrated in FIGURE 1. A major feature is that the DNA-binding domain is separate from the rest of the protein.
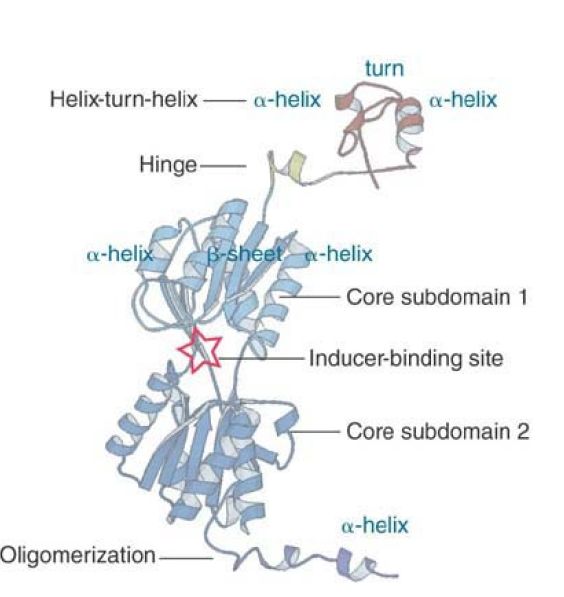
FIGURE 1 The structure of a monomer of the lac repressor identifies several independent domains.
Structure from Protein Data Bank 1LBG M. Lewis, et al., Science 271 (1996): 1247–1254. Photo courtesy of Hongli Zhan and Kathleen S. Matthews, Rice University.
The DNA-binding domain occupies residues 1–59. It contains two α-helices separated by a turn. This is a common DNA-binding motif known as the HTH (helix-turn-helix); the two α-helices fit into the major groove of DNA, where they make contacts with specific bases . This region is connected by a hinge sequence to the main body of the protein. In the DNA-binding form of the repressor, the hinge forms a small α-helix (as shown in Figure 1), but when the repressor is not bound to DNA this region is disordered. The HTH and hinge are sometimes referred to as the headpiece. The remainder of the protein is called the core. The bulk of the core consists of two interconnected regions with similar structures (core subdomains 1 and 2). Each has a six-stranded parallel β-sheet sandwiched between two α-helices on either side. The inducer binds in a cleft between the two regions. Two monomer core domains can associate to form a dimeric version of LacI.
Dimeric LacI tightly binds operator DNA because it recognizes both halves of the operator sequence, which is an inverted repeat (described shortly).
The C-terminus of the monomer contains an α-helix with two leucine heptad repeats. This is the tetramerization domain. The tetramerization helices of four monomers associate to maintain the tetrameric structure. FIGURE 2 shows the structure of the tetrameric core (using a different modeling system than Figure 1). It consists, in effect, of two dimers. The body of the dimer contains an interface between the subdomains of the two core monomers and two clefts in which two inducers bind (top). The Cterminal regions of each monomer protrude as helices. (The headpiece would join with the N-terminal regions at the top.) Together, the two dimers form a tetramer (center) that is held together by a C-terminal bundle of four helices.

FIGURE 2 The crystal structure of the core region of the lac repressor identifies the interactions between monomers in the tetramer. Each monomer is identified by a different color. Mutations are colored as follows: dimer interface = yellow; inducer binding =blue; oligomerization = white and purple. The protein orientation in the middle panel is rotated ~90° along the z-axis relative to the top panel.
Photos courtesy of Benjamin Wieder and Ponzy Lu, University of Pennsylvania.
FIGURE 3 shows a schematic for how the monomers are organized into the tetramer. Two monomers form a dimer by means of contacts at core subdomains 1 and 2; other contacts occur between their respective tetramerization helices. The dimer has two DNA-binding domains at one end of the structure and the tetramerization helices at the other end. Two dimers then form a tetramer by interactions at the tetramerization interface. Each tetramer has four inducer-binding sites and two DNA-binding sites.

FIGURE 3 The repressor tetramer consists of two dimers. Dimers are held together by contacts involving core subdomains 1 and 2 as well as by the tetramerization helix. The dimers are linked into the tetramer by the tetramerization interface.
Mutations in the lac repressor identified the existence of different domains even before the structure was known. The nature of the mutations can be described more fully by reference to the structure, as shown in FIGURE 4. Recessive mutations of the lacI type can occur anywhere in the bulk of the protein. Basically, any mutation that inactivates the protein will have this phenotype. The more detailed mapping of mutations onto the crystal structure in Figure 24.14 identifies specific impairments for some of these mutations—for example, those that affect oligomerization.

FIGURE 4 The locations of three types of mutations in lactose repressor are mapped on the domain structure of the protein. Recessive lacI mutants that cannot repress can map anywhere in the protein. Dominant negative lacI mutants that cannot repress map to the DNA-binding domain. Dominant lacI mutants that cannot induce because they do not bind inducer or cannot undergo the allosteric change map to core subdomain 1.
The special class of dominant negative lacI mutations lies in the DNA-binding site of the repressor subunit (see the section trans- Acting Mutations Identify the Regulator Gene earlier in this chapter). This explains their ability to prevent mixed tetramers from binding to the operator; reducing the number of binding sites reduces the specific affinity for the operator. The role of the Nterminal region in specifically binding DNA is also shown by the occurrence of “tight-binding” mutations in this region. These rare mutations increase the affinity of the repressor for the operator, sometimes so much that it cannot be released by inducer.
Uninducible lacI mutations map largely in a region of the core subdomain 1, extending from the inducer-binding site to the hinge. One group lies in amino acids that contact the inducer, and these mutations prevent binding of the inducer. The remaining mutations lie at sites that must be involved in transmitting the allosteric change in conformation to the hinge when the inducer binds.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|