

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Repair Systems Correct Damage to DNA
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المصدر:
LEWIN’S GENES XII
الجزء والصفحة:
19-4-2021
2297
Repair Systems Correct Damage to DNA
KEY CONCEPTS
- Repair systems recognize DNA sequences that do not conform to standard base pairs.
- Excision repair systems remove one strand of DNA at the site of damage and then replace it.
- Recombination-repair systems use homologous recombination to replace the double-stranded region that has been damaged.
- All these systems may introduce errors during the repair process.
- Photoreactivation is a nonmutagenic repair system that acts specifically on pyrimidine dimers.
- Methyltransferase enzymes can directly reverse alkylation damage in a suicide reaction.
The types of damage that trigger repair systems can be divided into three general classes: single-base changes, structural distortions/bulky lesions, and strand breaks.
Single-base changes affect the sequence of DNA but do not grossly distort its overall structure. They do not affect transcription or replication when the strands of the DNA duplex are separated.
Thus, these changes exert their damaging effects on future generations through the consequences of the change in DNA sequence. The reason for this type of effect is the conversion of one base into another that is not properly paired with the partner base. Single-base changes may happen as the result of mutation of a base in situ or by replication errors. FIGURE 1 shows that deamination of cytosine to uracil (spontaneously or by chemical mutagen) creates a mismatched U-G pair. FIGURE 2 shows that a replication error might insert adenine instead of cytosine to create an A-G pair. Similar consequences could result from covalent addition of a small group to a base that modifies its ability to base pair. These changes may result in very minor structural distortion (as in the case of a U-G pair) or quite significant change (as in the case of an A-G pair), but the common feature is that the mismatch persists only until the next replication. Thus, only limited time is available to repair the damage before it is made permanent by replication. This repair is mediated by a replication-linked mismatch repair system.
Structural distortions provide a physical impediment to replication or transcription. Introduction of covalent links between bases on one strand of DNA or between bases on opposite strands inhibits replication and transcription. FIGURE 3 shows the example of ultraviolet (UV) irradiation, which introduces covalent bonds between two adjacent pyrimidine bases (thymine in this example) and results in an intrastrand pyrimidine dimer, which can take the form of a cyclobutane pyrimidine dimer (CPD, as shown in Figure 3 ) or a 6,4 photoproduct (6,4PP). Of all the pyrimidine dimers, thymine–thymine dimers are the most common, and cytosine–cytosine dimers are the least common. In addition, while 6,4PPs are only about one-third as common as CPDs, they may be more mutagenic. These lesions can be repaired by photoreactivation in species that have this repair mechanism. This system is widespread in nature, occurring in all but placental mammals, and appears to be especially important in plants. In E. coli it depends on the product of a single gene (phr) that encodes an enzyme called photolyase. (Placental mammals repair these lesions via excision repair, as described below.)
FIGURE 4 shows that similar transcription- or replicationblocking consequences can result from the addition of a bulky adduct to a base that distorts the structure of the double helix. In this example, aberrant methylation of guanine results in a lesion that prevents normal base pairing. O6-methylguanine (O6-meG) is a common mutagenic lesion that can be repaired in several ways.
O6-meG is actually a substrate for one of the direct repair pathways: The protein O6-methylguanine DNA methyltransferase (MGMT) directly transfers the methyl group from O6-meG to a cysteine in MGMT, restoring guanine, as shown in FIGURE 5.
This is a suicide reaction, in that the methylated MGMT cannot regenerate a free cysteine; instead it is degraded after the repair process.
The loss or removal of a base to create an abasic site, as shown in FIGURE 6 , prevents a strand from serving as a proper template for synthesis of RNA or DNA. Abasic sites are repaired by excision repair via removal of the phosphodiester backbone where the base is missing.
DNA strand breaks can occur in one strand or both. A single-strand break, or nick, can be directly ligated. DSBs are a major class of damage that, if unrepaired, can result in extensive loss of DNA. The common feature in all these changes is that the damaged adduct (or break) remains in the DNA and continues to cause structural problems and/or induce mutations until it is removed.
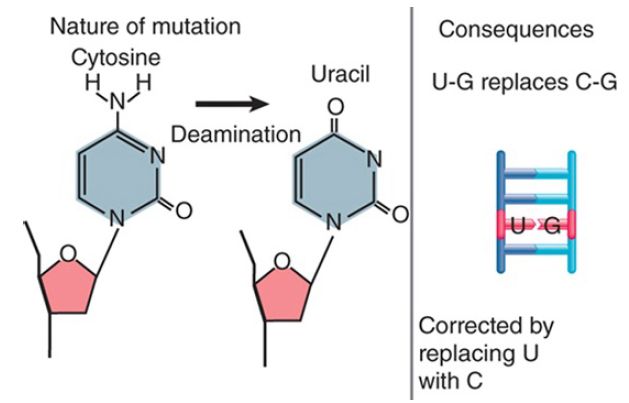
FIGURE 1. Deamination of cytosine creates a U-G base pair. Uracil is preferentially removed from the mismatched pair.
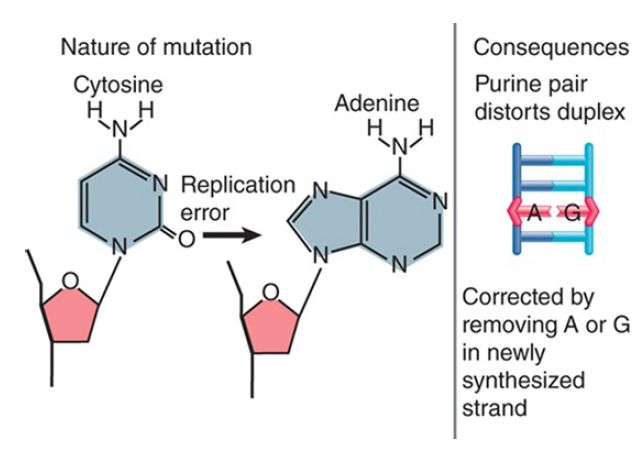
FIGURE 2 A replication error creates a mismatched pair that may be corrected by replacing one base; if uncorrected, a mutation is fixed in one daughter duplex.
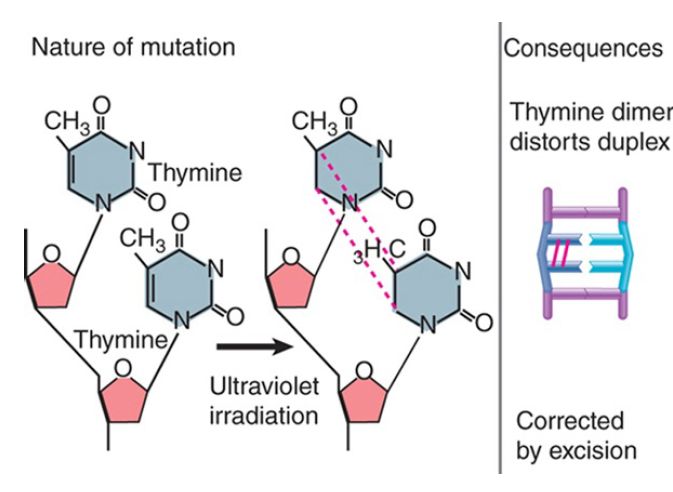
FIGURE 3 Ultraviolet irradiation causes dimer formation between adjacent thymines. The dimer blocks replication and transcription.
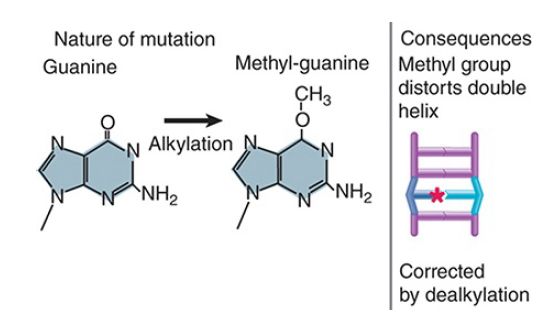
FIGURE 4 Methylation of a base distorts the double helix and causes mispairing at replication. Star indicates the methyl group.
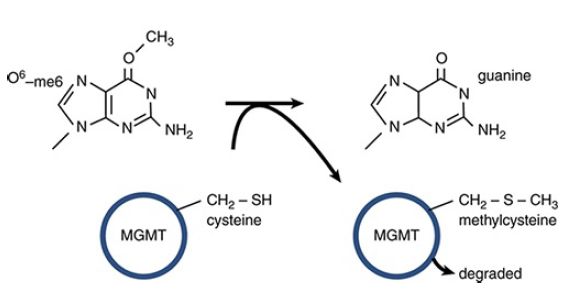
FIGURE 5 MGMT can directly transfer a methyl group from O6-meG to a cysteine residue in the protein. This restores guanine but is an irreversible reaction that results in inactivation and degradation of MGMT.
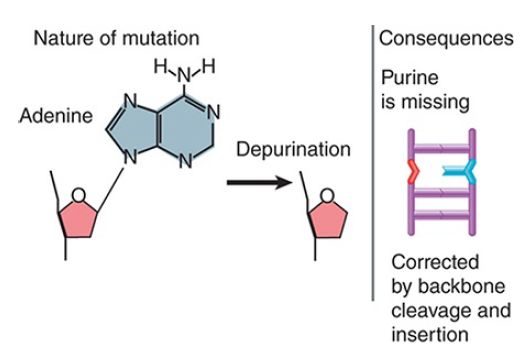
FIGURE 6 Depurination removes a base from DNA, blocking replication and transcription.
When a repair system is eliminated, cells become exceedingly sensitive to agents that cause DNA damage, particularly the type of damage recognized by the missing system. The importance of these systems is also emphasized by the fact that mutation of repair genes is associated with the development of a number of cancers in humans, such as Lynch syndrome (also called hereditary nonpolyposis colorectal cancer, or HNPCC), caused by defects in mismatch repair.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















