


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 25-2-2021
Date: 15-12-2015
Date: 15-5-2016
|
Synthetases Use Proofreading to Improve Accuracy
KEY CONCEPT
- Specificity of amino acid–tRNA pairing is controlled by proofreading reactions that hydrolyze incorrectly formed aminoacyl adenylates and aminoacyl-tRNAs.
Aminoacyl-tRNA synthetases must distinguish one specific amino acid from the cellular pool of amino acids and related molecules and must also differentiate cognate tRNAs in a particular isoaccepting group (typically one to three) from the total set of tRNAs. tRNA discrimination can be successfully accomplished based on detailed differences in the L-shaped structures . This occurs at both the initial binding step and at the level of induced fit; noncognate tRNAs derived from other isoaccepting groups lack the full identity set of nucleotides and are consequently unable to rearrange their structure to adopt an enzyme-bound conformation in which the reactive CCA terminus is properly aligned with the amino acid carboxylate group and the ATP α-phosphate. This rejection of noncognate tRNAs at a stage of the reaction that precedes the synthesis of misacylated tRNA is sometimes referred to as kinetic proofreading. The inability of noncognate tRNAs to proceed through the chemical steps of aminoacylation arises because the tRNA dissociates from the enzyme much faster than it can react (FIGURE 1).
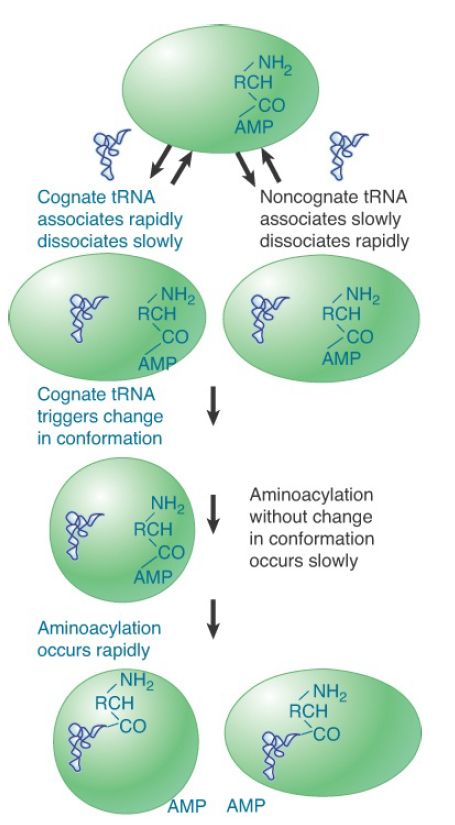
FIGURE 1. Aminoacylation of cognate tRNAs by synthetase is based, in part, on greater affinities for these types, coupled with weak affinities for noncognate types. In addition, noncognate tRNAs are unable to fully undergo the induced-fit conformational changes required for the later catalytic steps.
In contrast, tRNA synthetases are unable to distinguish between some structurally similar amino acids in the course of the two-step aminoacyl-tRNA synthesis reaction alone. It is especially difficult for the enzymes to distinguish between two amino acids that differ only in the length of the carbon backbone (i.e., by one –CH group), or between amino acids of the same size that differ at only one atomic position. For example, the amino acid–binding pocket of isoleucyl-tRNA synthetase (IleRS) cannot distinguish isoleucine from valine sufficiently well enough to prevent synthesis of a significant amount of Val-tRNAIle . Similarly, valyl-tRNA synthetase (ValRS) synthesizes Thr-tRNAVal to a significant extent.
IleRS, ValRS, and at least seven additional tRNA synthetases (those specific to leucine, methionine, alanine, proline, phenylalanine, threonine, and lysine) are able to correct, or proofread, the aminoacyl adenylates and aminoacyl-tRNA formed in their active sites by means of additional activities that either hydrolyze the aminoacyl-AMP to yield free amino acid and AMP or that hydrolyze the misacylated tRNA to yield free amino acid and deacylated tRNA. The hydrolysis of aminoacyl-AMP is referred to as pretransfer editing, whereas the hydrolysis of aminoacyl-tRNA is referred to as posttransfer editing (FIGURE 2). In the case of
pretransfer editing, it is also possible that some of the incorrectly formed aminoacyl-AMP dissociates from the active site, after which it is hydrolyzed nonenzymatically in solution (the aminoacyl ester bond is relatively unstable). This type of editing reaction can also be considered as a form of kinetic proofreading. In contrast, pretransfer hydrolysis of noncognate aminoacyl adenylate when bound by the enzyme, as well as enzyme-catalyzed posttransfer editing, are each known as chemical proofreading. Although pretransfer editing reactions may sometimes occur in the absence of tRNA (i.e., before tRNA binding), the presence of tRNA generally substantially improves the efficiency of the hydrolytic reaction. The extent to which pretransfer versus posttransfer editing predominates varies with the individual synthetase.
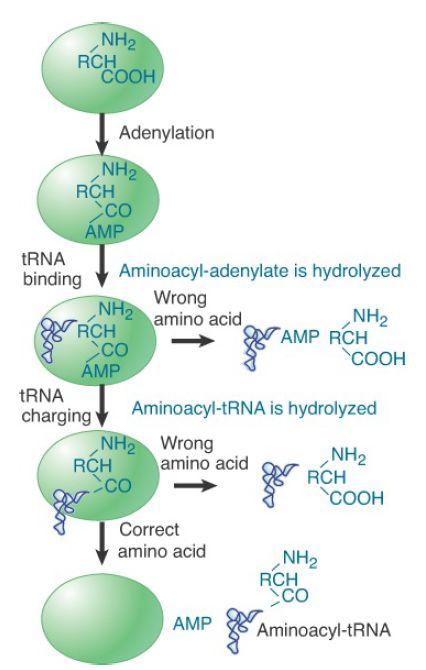
FIGURE 2. Proofreading by aminoacyl-tRNA synthetases may take place at the stage prior to aminoacylation (pretransfer editing), in which the noncognate aminoacyl adenylate is hydrolyzed. Alternatively or additionally, hydrolysis of incorrectly formed aminoacyl-tRNA may occur after its synthesis (posttransfer editing).
A general way to think of the editing reaction is in terms of the classic double-sieve mechanism, illustrated for IleRS in FIGURE 3, in which the size of the amino acid is used as the basis for discrimination. IleRS possesses two active sites: the synthetic (or activation) site located in the common class I Rossmann-fold domain and the editing (or hydrolytic) site located in the acceptorbinding domain (see the earlier section, Aminoacyl-tRNA Synthetases Fall into Two Classes). The crystal structure of IleRS shows that the synthetic site is too small to allow leucine to enter (the leucine side-chain is branched at a different position as compared with isoleucine). Indeed, all amino acids larger than isoleucine are excluded from activation because they cannot enter the synthetic site. However, some smaller amino acids that retain sufficient capacity to bind—such as valine—can enter the synthetic site and become attached to tRNA. The synthetic site functions as the first sieve. The editing site is smaller than the synthetic site and cannot accommodate the cognate isoleucine, but it does bind valine. Thus, Val-tRNAIle can be hydrolyzed in the editing site, functioning as the second sieve, while Ile-tRNAIle is not hydrolyzed.
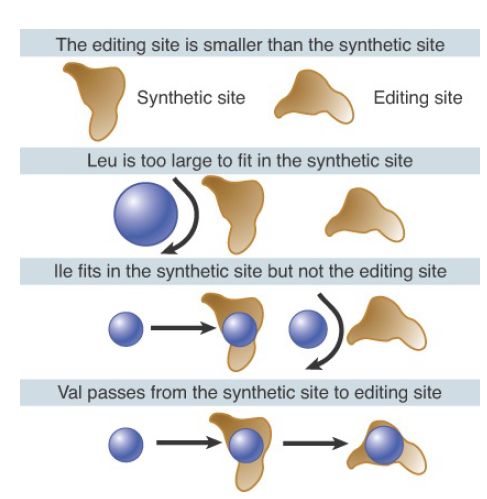
FIGURE 3. Isoleucyl-tRNA synthetase has two active sites. Amino acids larger than Ile cannot be activated because they do not fit in the synthetic site. Amino acids smaller than Ile are removed because they are able to enter the editing site.
The double-sieve model functions as a convenient and generally accurate way to think of posttransfer editing. In IleRS, as well as in other editing tRNA synthetases from both class I and class II, the synthetic and editing sites are located a considerable distance apart, on the order of 10 to 40 Å. For posttransfer hydrolysis (editing) to occur, the misacylated aminoacyl-tRNA acceptor end is translocated across the surface of the enzyme, moving from the synthetic site to the editing site. This involves a change in the conformation of the acceptor end of the tRNA. In class I tRNA synthetases, the acceptor end adopts a hairpinned conformation when bound in the synthetic site (see the earlier section, Aminoacyl-tRNA Synthetases Fall into Two Classes) and an extended structure when bound in the editing site.
Translocation of the incorrect amino acid across the tRNA synthetase surface in posttransfer editing is possible because it is covalently bound to the 3′ end of the tRNA. In contrast, pretransfer editing occurs before formation of the aminoacyl-tRNA bond, and this reaction is instead localized within the confines of the synthetic active site. Kinetic partitioning of the aminoacyl-adenylate intermediate between hydrolysis and aminoacyl transfer may control the extent to which an editing tRNA synthetase relies on pretransfer versus posttransfer editing.



|
|
|
|
مخاطر عدم علاج ارتفاع ضغط الدم
|
|
|
|
|
|
|
اختراق جديد في علاج سرطان البروستات العدواني
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|