

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Growth Factors
المؤلف:
D. Gospodarowicz, A. Baird, J. Cheng, G. M. Lui, F. Esch, and P. Böhlen
المصدر:
Endocrinology 118, 82–90
الجزء والصفحة:
15-5-2016
4035
Growth Factors
Peptide growth factors provide a critical foundation for intercellular communication in multicellular organisms and are key elements in a complex biological process. But while they promote cell growth, just as often they can have opposite, inhibitory effects on cell proliferation, or instead modulate differentiated cell function. They regulate numerous alternative cell functions that are related to, but sometimes distinct from, cell proliferation. Their activities are often distinct, one from each other, but they can also be significantly redundant; alone, they may exert one activity, but in combination have different, even opposite, effects on cells. To understand their activities, their actions must be defined individually, and then evaluated in the context of complex biological responses.
There are a number of specific characteristics that define the specific properties of a protein growth factor. First and foremost, they are proteins, usually of molecular weights ranging from 5000 to 80,000 daltons. Second, they are ligands that modulate cell function through cell-surface high affinity receptors. On binding the ligand, the receptor activates a signal-transduction cascade that, when evaluated on cells in culture, stimulates a proliferative response that includes (but is not limited by) thymidine incorporation into DNA and cell division.
Different peptide growth factors belong to one or another family of structurally related proteins. Hence, numerous proteins that contain sequences homologous to epidermal growth factor (EGF( belong to the EGF family, others with homology to platelet derived growth factor (PDGF) belong to the PDGF family, and still others related to nerve growth factor (NGF), transforming growth factor-b (TGF b), hepatocyte growth factor (HGF) all belong to the NGF, PDGF, TGF b, or HGF family, respectively. A representative list of these families and some of their members is shown in Table 1.
Table 1. Growth Factor Families
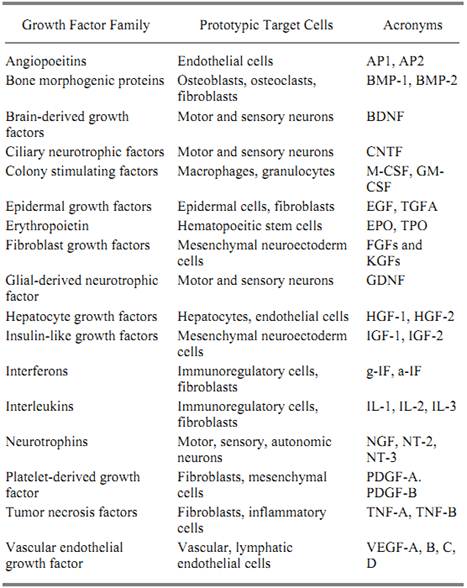
When peptide growth factors were first being isolated and identified, they were described either by their biological activity or by the source from which they were purified. Hence, the first fibroblast growth factors (FGFs) were isolated on the basis of their ability to stimulate the proliferation of fibroblasts, NGF by its ability to stimulate nerve cells, TGFs for their ability to transform cells, HGF for its ability to stimulate hepatocytes, and PDGF because it was first purified from platelets. However, the original discovery of a peptide growth factor has seldom reflected the extent of the protein's activities or, in fact, its true physiological function. Few recall that one of the most extensively studied growth factors, EGF, was first named urogastrone and was originally characterized for its ability to suppress gastric acid secretion (1). A powerful peptide growth factor in its own right, EGF is a potent growth factor for epithelial cells and yet, in 1962, was isolated and purified on the basis of its ability to cause premature eruption of incisor teeth in mice and not cell proliferation per se (2). Similarly, before the advent of modern cell culture, the detection, isolation, and identification of NGF in 1953 (3), 1960 (4) and 1971 (5), respectively, were based on in vivo
studies and tissue explants that showed increased neurite outgrowth from nerve ganglia. But is NGF a true nerve growth factor when it stimulates neurite outgrowth, but not nerve cell proliferation? Even more importantly, NGF can stimulate hematopoietic cell colony growth and differentiation (6) and has receptors on the cell surface of fibroblasts, lymphocytes, and numerous mesenchymal cell types that are unrelated to cells of neural lineage (7, 8). The detection of both ligand and receptor in reproductive tissues points to possible functions in reproduction.
In the late 1970s, and throughout the 1980s, the advent of modern methods of cell biology gave investigators the ability to grow numerous cell types in culture, and there was a flurry of activity to define the conditions in which each cell type could grow (9). The first efforts were aimed at replacing serum with tissue extracts so as to identify the active components. Hence, the growth factor literature became saturated with descriptions of “activities” known as fibroblast growth factors, endothelial cell growth factors, cartilage growth factors, milk-derived growth factors, adrenal angiogenic factor, corpus luteum growth factor, brain growth factor, pituitary growth factor, to name only a few. On applying the then developing techniques of molecular cloning, it soon became apparent that all of these seemingly disparate activities were in fact a handful of molecules: FGFs, EGFs, HGFs and PDGFs.
With the realization that, historically, peptide growth factors are a loosely defined collection of molecules that promote cell growth, there have been numerous efforts to rename the field, reorganize its nomenclature, and refine the description of peptide growth factors. While cell biologists have named these molecules “growth factors,” immunologists have called them “interleukins, lymphokines, and cytokines”. Hematologists, in turn, have coined the phrase colony stimulating factors. All descriptions are found throughout the literature and vary from field to field. In the end, the most common usage has remained the historical names given to molecules as they have been identified. When molecules are “rediscovered” because of a new heretofore unknown activity, they are classified according to their original, and ironically sometimes their least important, activity. Hence, when the adrenal angiogenic factor was sequenced and found to be identical to FGF2, it was renamed as such (10). When keratinocyte growth factor (KGF) was sequenced and found to be a member of the FGF family, it was renamed FGF7 (11). As the field has matured, and more structural and biological information has been generated, it has been possible to sort through the pleiotropic activities of growth factors and their sometimes ubiquitous distribution, to rely more heavily on their structural, rather than biological, characteristics for classification (12).
The difficulty in classifying growth factors and the attempts to distinguish them from interleukins, lymphokines, and cytokines is futile and best illustrated by an examination of their range of target cells. It has already been noted that any given growth factor can have numerous activities on any given cell type and that their activities can expand well beyond the context of their original discovery. Even more remarkable, however, is their range of target cells, which can be much greater than originally anticipated. It is here where attempts to delineate functional differences between interleukins, cytokines, and growth factors have failed. Interleukins and cytokines are generally regarded as signaling molecules with activities in the immune system. They were originally thought to be produced by the immune system, for the immune system. Yet many, if not all, interleukins are broad-spectrum proteins that, when studied in detail, regulate numerous processes outside of the immune system. Interleukin 1 (IL1), for example, acts on chondrocytes, astrocytes, fibroblasts, keratinocytes, and some neurons. Best known as a B-cell growth factor, interleukin-6 also regulates mesenchymal cell function. Perhaps even more remarkable is the observation that fibroblasts and other nonimmune cells can be sources of interleukins and not just targets, thus diminishing any differences between interleukins and protein growth factors further. As pleiotropic, ubiquitous biological effectors that control cell proliferation, the terms protein growth factor, cytokine, and interleukin describe near synonymous molecules.
Whatever classification might be conferred to growth factors, cytokines, lymphokines, or interleukins, their activities challenge the foundations of classical sciences like endocrinology, because of their peculiar, and yet necessary, mode of action. Unlike the endocrine system that relies on tissue-to-tissue communication, the growth factor system acts at the cellular level. Thus, while the endocrine pathways rely on blood-borne transportation of signaling molecules to effect change, growth factors mediate alterations in the local biological milieu. This fundamental difference in action has led investigators to rethink how cells communicate and even how they respond to endocrine stimuli. Peptide growth factors, released into the local milieu by one cell, alter the activity of an adjacent cell that is in close proximity (paracrine target) or alternatively the cell from which it derives (autocrine target). The actions of major regulatory peptides are mediated by autocrine and paracrine mechanisms that control growth factors and normal cellular homeostasis. Thus the main difference between the growth factor and cytokine pathways and the endocrine system is in the local action of growth factors. Even lymphokines produced by circulating immune cells act locally, but only after the cell producing it (eg, lymphocytes) has traveled and delivered it to its desired site of action. All growth factors are produced locally to act locally.
With this difference between endocrine and growth factor pathways in mind, it is possible to understand that when growth factors are locally released into biological fluids, they can then act to modify the actions of classic hormones arriving on site from distal sources. Hormone action has long been known to require local cellular factors to permit, mediate, and sometimes modulate responsiveness. For example, insulin-like growth factors and their binding proteins modulate hormone action (13) and TGF-b can modulate adrenal function (14). Furthermore, endocrine hormones can trigger gene expression of growth factors in target cells, which in turn modulate the local milieu and change the cell's responsiveness to further stimulation by the hormone (15).
In view of the fact that peptide growth factors act locally to modulate cell function, it comes as no surprise that there are numerous processes present in the local milieu to control their activities and ensure that their actions do not extend to unwanted targets. Of these processes, four are perhaps the most studied:
• Restricted production and release of the growth factor to limit its action
•Highly regulated receptor gene expression to limit target cell responsiveness
•Circulating inhibitors in blood to sequester and inactivate the ligand
• The binding and sequestration of growth factors at the cell surface and extracellular matrix to keep them in the local milieu
The notion that there is restricted production and release of growth factors and limited receptor expression is best illustrated by the difference between gene expression in embryonic development, fetal growth, and in adult tissues (16). At times as early as before gastrulation, the gene expression of growth factors becomes controlled in both a temporal and spatial fashion, and the role played by growth factors in development is essential in all aspects of patterning, tissue development, and differentiation. As exemplified by studies where the genes for different growth factors have been genetically knocked out, they have been shown to play a critical role during development, but are often suppressed in adult, quiescent tissues. In both cancer and wound healing, however, the genes for these same molecules are activated. In wound healing, they remain regulated until the injury has been resolved. In cancer, activation of these genes leads to uncontrolled cell growth, transformation, abnormal blood vessel growth, and ultimately tumor formation.
One particularly interesting mechanism of controlling growth factor activity appears unique to some of the paracrine factors involved in the injury and inflammatory response. Recent data suggest that molecules like FGF1, FGF2, IL1a, and Il-b can exit the cell independently of the cell's secretory endoplasmic reticulum and Golgi apparatus (17). This protein export process is mechanistically different from protein secretion and is highly specific and regulated. While its physiological significance remains unknown, the selective translocation of certain biological effectors across the plasma membrane presumably restricts their activity and allows the cell to tightly control access to the cell surface and their target cells.
The evidence for natural inhibitors of growth factors is equally compelling. For example, numerous investigators have established that genes encoding high affinity receptors for growth factors encode soluble forms of their high affinity receptors that are generated by alternative splicing of the gene's mRNA or by truncation at the cell surface (18). This form of receptor has no transmembrane domain and no signaling domain. It is secreted by cells and can bind ligand in biological fluids, thus preventing it from interacting with its cell surface (and functional) high affinity receptor. Alternatively, some cells have the ability to produce receptor antagonists that bind, but do not activate high affinity receptors. By virtue of occupying the binding site, molecules like interleukin receptor antagonist (ILRA) and angiopoietin-2 prevent a functional interaction between the ligand and its native receptor.
Perhaps the most remarkable process that appears to control the activity of growth factors stems from the observation that they often appear designed to remain in the local cellular milieu and physically sequestered outside target cells in what is presumed to be a biologically inactive form . (19) This observation underlies the need for their action to remain local and predicts the existence of specific signals (eg, enzymes) to render them available to target cells.
The first type of local adhesion is characterized by a noncovalent association of growth factors with the extracellular matrix, the cell surface, or pericellular structures. This binding is often salt- and/or pH-sensitive. Representative examples of growth factors with this type-1 adhesion are shown in Table 2. In contrast, the second type of adhesion is characterized by a covalent association with the extracellular surface, through a transmembrane domain, in the growth factor's precursor. Representative examples of growth factors having this type 2 adhesion are also shown in Table 2.
Table 2. Growth Factors Associated with Cell Surface through Ionic (Type 1) or Transmembrane Domains (Type 2) in Their Precursors
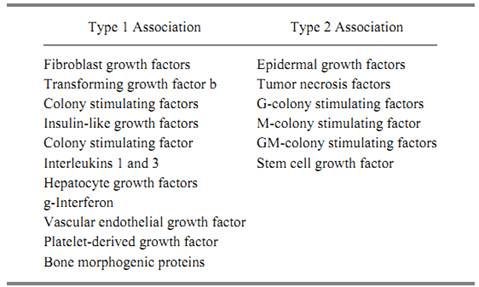
Fibroblast growth factors (FGFs) are prototypic examples of type 1 adhesion. They have a high affinity for immobilized heparin, and immunohistochemical techniques localize FGF1 and FGF2 to the extracellular matrix, basement membrane, and cell surface of numerous tissues (20). Indeed, recent studies have identified the binding heparan sulfate proteoglycans that serve to sequester (and possibly deliver) FGFs to its high affinity receptors on target cells. TGFb has also been localized in extracellular and pericellular structures, but it is in what is thought to be a latent, biologically inert form (21). Recent studies have identified three kinds of receptors for this protein, one of which is a
high molecular weight proteoglycan anchored onto the plasma membrane through phosphoinositol. Other growth factors with this type 1 adhesion include granulocyte-macrophage colony stimulating factor (GM-CSF), which binds to extracellular matrix and to heparan sulfate-related glycosaminoglycans (GAG), interleukin-3 (IL-3), hepatocyte growth factor (HGF), osteogenin ) OTG), and the bone morphogenic proteins (BMP).
Type 2 adhesion is characterized by growth factors that are covalently associated with the plasma membrane because their precursor encodes a transmembrane domain that anchors it to the cell surface. In order to be active, these molecules must either be processed from the precursor to generate an active ligand or must act directly on an adjacent high affinity receptor without being further processed or released. Accordingly, these molecules may have activities that are restricted by availability. The prototypic family (and the most studied) of the growth factors with type 2 adhesion to cells is EGF. It and its structural homologues, TGFa and the heparin-binding EGF, are contained in large precursor proteins that have transmembrane domains that lock them onto the cell surface. Most of the biological studies performed with EGFs have been with the processed unbound forms, so it is less clear if their growth factor activities are maintained when they are unprocessed, at the cell surface. But recent studies using site-directed mutagenesis support the notion that the membrane-bound precursor has growth factor activity and can interact with high affinity receptors on target cells. The ability of growth factors with this type 2 adhesion to elicit a biological response is illustrated by the recent experiment showing that PDGF anchored to the plasma membrane through mutagenesis is still capable of stimulating a mitogenic response (22). Other examples of type 2 adhesion include tumor necrosis factor (TNF) and the hematopoietic factor, CSF-1 and stem cell growth factor, the ligand for the ckit protein. In all instances, each is generated from an integral membrane protein locked onto the cell surface.
It is presumed that a growth factor locked to the cell surface through a transmembrane domain can stimulate a biological response only if it is liberated from its sites of sequestration and delivered to the target cell. The molecular mechanisms that might mediate this regulation are unknown. The extracellular matrix and the various constituents that bind the growth factor are obvious targets for the combined actions of proteolytic and glycolytic degradation. These include plasmins, cathepsins, collagenases, and a wide array of glycanases, such as heparinases and heparitinases. These enzymes are normally inactive due to the presence of a macroglobulin, plasminogen activator inhibitors, proteinases like nexin, and collagenase inhibitors. In the case of the FGFs, however, it is particularly worthy to note that they are proteinase-resistant when bound to glycosaminoglycans (23). Thus the generation of an FGF-GAG complex by limited proteolysis provides a mechanism to generate a potent biologically active ligand from a pool of sequestered, biounavailable growth factor. Furthermore, glycosaminoglycan binding to some FGFs has been reported to enhance its binding to their high affinity receptor and in some instances is required for delivery (24). The mechanisms that regulate the type 2 adhesion of growth are not known, although there exist proteolytic enzymes capable of releasing the ligand from the cell (25, 26).
In the end, as the peptide growth factors that control cell growth and development have been identified and the mechanisms that regulate their activities better understood, the challenge becomes to characterize how they all act together to control normal cell function. As the use of modern molecular techniques to eliminate combinations of growth factors from the genome genetically becomes more widespread, it should be possible to provide important insight into how these factors act to control normal cell function and, ultimately, their role in whole-body homeostasis.
References
1. H. Gregory and I. R. Wilshire (1975) Physiol. Chem. 356, 1765–1774.
2. S. Cohen (1962) J. Biol. Chem. 237, 1555–1562.
3. R. Levi-Montalcini and V. Hamburger (1952) J. Exp. Zool. 123, 233–288.
4. S. Cohen (1960) Proc. Natl. Acad. Sci. USA 46, 302–311.
5. R. H. Angeletti and R. A. Bradshaw (1971) Proc. Natl. Acad. Sci. USA 68, 2417–2420.
6. H. Matsuda, M. D. Coughlin, J. Bienstock, and J. A. Denburg (1988) Proc. Natl. Acad. Sci. USA 85, 6508–6512.
7. P. Ernfors, F. Hallbook, T. Ebendal, E. M. Shooter, M. J. Radeke, T. P. Misko, and H. Persson (1988) Neuron 1, 983–996.
8. M. Bothwell, S. L. Patterson, G. C. Schatteman, S. Thompron et al. (1989) J. Neurosci. 22, 354–362.
9. A. Baird (1993) Endocrinology 132, 487–488.
10. D. Gospodarowicz, A. Baird, J. Cheng, G. M. Lui, F. Esch, and P. Böhlen (1986( Endocrinology 118, 82–90.
11. P. W. Finch, J. S. Rubin, T. Miki, D. Ron, and S. A. Aaronson (1989) Science 245, 752–755.
12. A. Baird and M. Klagsbrun (1991) Cancer Cells 3, 239–243.
13. G. Lamson, L. C. Giudice, and R. C. Rosenfeld (1991) Growth Factors 5, 19–28.
14. J. J. Feige and A. Baird (1992) Prog. Growth Factor Res. 3, 103–113.
15. J. Massague (1990) Annu. Rev. Cell Biol. 6, 597–640.
16. M. Goldfarb (1996) Cytokine Growth Factor Rev. 7, 311–325.
17. A. E. Cleves (1997) Curr. Biol. 7, 318–320.
18. D. E. Johnson, J. Lu, H. Chen, S. Werner, and L. T. Williams (1991) Mol. Cell Biol. 11, 4627–4634.
19. J.-J. Feige and A. Baird (1992) Med. Sci. 8, 805–810.
20.I. Vlodavsky, J. Folkman, R. Sullivan et al. (1987) Proc. Natl. Acad. Sci. USA 84, 2292–2296.
21. N. L. Thompson, K. C. Flanders, J. M. Smith, L. R. Ellingsworth, A. B. Roberts, and M. B. Sporn (1989) J. Cell Biol. 108, 661–669.
22. B. A. Lee and D. J. Donoghue (1991) J. Cell Biol. 113, 361–370.
23. D. Gospodarowicz and J. Cheng (1986) J. Cell Physiol. 128, 475–484.
24. A. Yayon, M. Klagsbrun, J. D. Esko, P. Leder, and D. M. Ornitz (1991) Cell 64, 841–848.
25. J. Teixidó, R. Gilmore, D. C. Lee, and J. Massagué (1987) Nature 326, 883–885.
26. A. Pandiella and J. Massagué (1991) Proc. Natl. Acad. Sci. USA 88, 1726–1730.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















