


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 12-5-2016
Date: 6-11-2020
Date: 25-4-2016
|
Viral DNA Integrates into the Chromosome
KEY CONCEPTS
- The organization of proviral DNA in a chromosome is the same as a transposon, with the provirus flanked by short direct repeats of a sequence at the target site.
- Linear DNA is inserted directly into the host chromosome by the retroviral integrase enzyme.
- Two base pairs of DNA are lost from each end of the retroviral sequence during the integration reaction.
The organization of the integrated provirus resembles that of the linear DNA. The LTRs at each end of the provirus are identical. The 3′ end of U5 consists of a short inverted repeat relative to the 5′ end of U3, so the LTR itself ends in short inverted repeats. The integrated proviral DNA is like a transposon: The proviral sequence ends in inverted repeats and is flanked by short direct repeats of target DNA.
The provirus is generated by directly inserting a linear DNA into a target site. In addition to linear DNA, circular forms of the viral sequences also occur. One has two adjacent LTR sequences generated by joining the linear ends. The other has only one LTR—presumably generated by a recombination event and actually comprising the majority of circles. For a long time it appeared that the circle might be an integration intermediate (by analogy with the integration of lambda DNA). It is now known, though, that the linear form is used for integration.
Integration of linear DNA is catalyzed by a single viral product, the integrase. The integrase acts on both the retroviral linear DNA and the target DNA. The reaction is illustrated in FIGURE 1.
The ends of the viral DNA are important, just as they are for transposons. The most conserved feature is the presence of the dinucleotide sequence CA close to the end of each LTR. This CA
dinucleotide is conserved among all retroviruses, viral retrotransposons, and many DNA transposons as well. The integrase brings the ends of the linear DNA together in a ribonucleoprotein complex and then converts the blunt ends into recessed ends by removing the bases beyond the conserved CA. In general, this involves a loss of two bases.
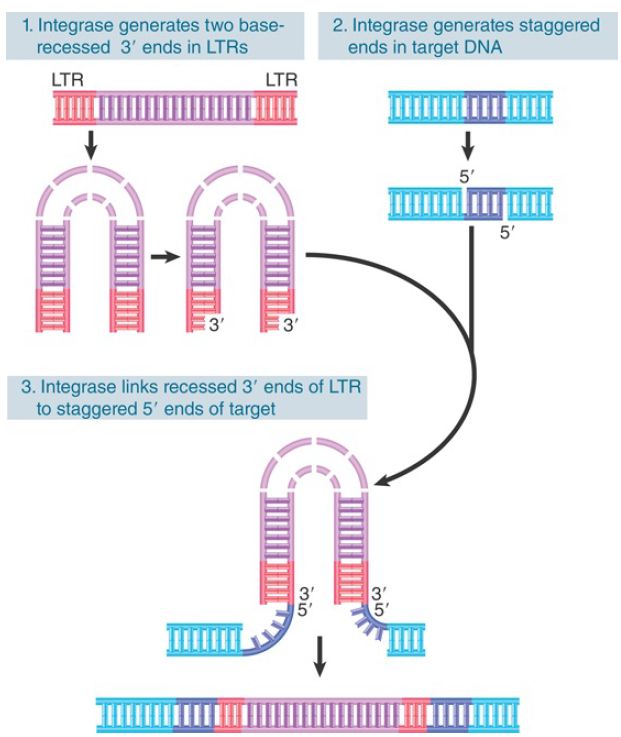
FIGURE 1. Integrase is the only viral protein required for the integration reaction, in which each LTR loses 2 bp and is inserted between 4-bp repeats of target DNA.
Target sites are chosen at random with respect to sequence. The integrase makes staggered cuts at a target site. In the example of Figure 1, the cuts are separated by 4 bp. The length of the
target repeat depends on the particular virus; it may be 4, 5, or 6 bp. Presumably, it is determined by the geometry of the reaction of integrase with target DNA.
The 5′ ends generated by the cleavage of target DNA are covalently joined to the 3′ recessed ends of the viral DNA. At this point, both termini of the viral DNA are joined by one strand to the target DNA. The single-stranded region is repaired by enzymes of the host cell, and in the course of this reaction the protruding two bases at each 5′ end of the viral DNA are removed. The result is that the integrated viral DNA has lost 2 bp at each LTR; this corresponds to the loss of 2 bp from the left end of the 5′ terminal U3 and to the loss of 2 bp from the right end of the 3′ terminal U5.
There is a characteristic short direct repeat of target DNA at each end of the integrated retroviral genome. The viral DNA integrates into the host genome at randomly selected sites. A successfully infected cell gains 1 to 10 copies of the provirus. An infectious virus enters the cytoplasm, of course, but the DNA form becomes integrated into the genome in the nucleus. Some retroviruses can replicate only in proliferating cells, because entry into the nucleus requires the cell to pass through mitosis, when the viral genome gains access to the nuclear material.
Others, such as HIV, can be actively transported into the nucleus even in the absence of cell division. The U3 region of each LTR carries a promoter. The promoter in the left LTR is responsible for initiating transcription of the provirus. Recall that the generation of proviral DNA is required to place the U3 sequence at the left LTR; thus, we see that the promoter is in fact generated by the conversion of the RNA into duplex DNA. Sometimes (probably rather rarely), the promoter in the right LTR sponsors transcription of the host sequences that are adjacent to the site of integration. The LTR also carries an enhancer (a sequence that activates promoters in the vicinity) that can act on cellular as well as viral sequences. Integration of a retrovirus can be responsible for converting a host cell into a tumorigenic state when certain types of genes are activated in this way.
We have dealt thus far with retroviruses in terms of the infective cycle, in which integration is necessary for the production of further copies of the RNA. When a viral DNA integrates in a germline cell, though, it becomes an inherited “endogenous provirus” of the organism. Endogenous viruses usually are not expressed, but sometimes they are activated by external events, such as infection with another virus.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تقدم دعوة إلى كلية مزايا الجامعة للمشاركة في حفل التخرج المركزي الخامس
|
|
|