


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 14-11-2020
Date: 4-5-2021
Date: 11-3-2021
|
DNA Microarrays
KEY CONCEPTS
-DNA microarrays comprise known DNA sequences spotted or synthesized on a small chip.
-Genome-wide transcription analysis is performed using labeled cDNA from experimental samples hybridized to a microarray containing sequences from all ORFs of the organism being used.
-Single nucleotide polymorphism arrays permit genomewide genotyping of single-nucleotide polymorphisms.
-Array-comparative genomic hybridization allows the detection of copy number changes in any DNA sequence compared between two samples.
A logical technical progression from Southern and northern blotting is the microarray. Instead of having the unknown sample on the membrane and the probe in solution, this effectively reverses the two. These originated in the form of “slot-blots” or “dot-blots,” whereby a researcher would spot individual DNA sequences of interest directly onto a hybridization membrane in an ordered pattern, with each spot consisting of a different, single, known sequence. Drying of the membrane immobilized these spots, creating a premade blotting array. In use, the researcher would then take a nucleic acid sample of interest, such as total cellular DNA, and then fragment and randomly and uniformly label this DNA (originally with a radioisotopic label). This labeled mix of sample DNA could then be used exactly as in a Southern blot as a probe to hybridize to the premade blot. Labeled DNA sequences homologous to any of the array spots would hybridize and be retained in the known, fixed location of that spot and be visualized by autoradiography. By viewing the autoradiogram and knowing the physical location of each specific probe spot, the pattern of hybridized versus nonhybridized spots could be read out to indicate the presence or absence of each of the corresponding known sequences in the unknown sample.
Technological improvements to this approach followed rapidly through miniaturization of the size and physical density of the immobilized spots, going from membranes with 30 to 100 spots to glass microscope slides with up to 1,000 spots. Today, silicon chip substrates have hundreds of thousands and up to a million or more individual spots in an area about the size of a postage stamp.
To visualize the distinct spots in such a high-density array, automated optical microscopy is used and fluorescence has replaced radiolabeling both to allow for increased spatial resolution (higher spot density) and easier quantification of each hybridization signal. In parallel with the increased total number of spots per array, the length of each unique probe has generally become shorter, allowing for each spot in the array to be specific to a smaller target area—in effect, giving greater “resolution” on a molecular scale. Although the potential applications of microarrays are really limited only by the user’s imagination, there are a number of particular applications for which they have become standard tools.
The first of these is in gene expression profiling, wherein a total mRNA sample from a specimen of interest (e.g., tissue in a disease state or under a particular environmental challenge) is collected and converted en masse to cDNA by a random primed reverse transcription. A label is incorporated into the cDNA during its synthesis (either through use of labeled nucleotides or having the primers themselves with a label); this can be either a fluorophore (“direct labeling”) or another hapten (such as biotin), which can at a later stage be exposed to a fluorophore conjugate that will bind the hapten (in the present example, streptavidin–phycoerythrin conjugate might be used) in what is called “indirect labeling.” This labeled cDNA is then hybridized to an array where the immobilized spots consist of complementary strands to a number of known mRNAs from the target organism. Hybridization, washing, and visualization allow for the detection of those spots that have bound their complementary labeled cDNA and thus the readout of which genes are being expressed in the original sample.
This process is depicted in FIGURE 1. This method is fairly quantitative, meaning that the observed signal on each spot corresponds reasonably well to the original level of its particular mRNA. Clever selection of the sequence of each of the immobilized spots, such as choosing short probe sequences that are complementary to particular alternate exons of a gene, can even
allow the method to differentiate and quantitate the relative levels of alternate splicing products from a single gene. By comparison of the data from such experiments performed in parallel on
experimental tissue and control tissue, an experiment can collect a snapshot of the total cellular “global” changes in gene expression patterns, often with useful insight into the state or condition of the experimental tissue.
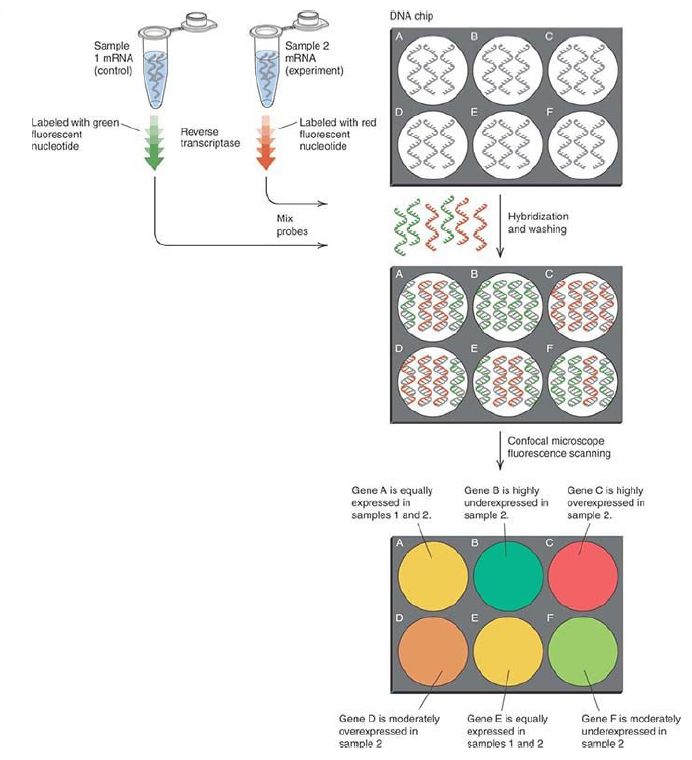
FIGURE 1. Gene expression arrays are used to detect the levels of all the expressed genes in an experimental sample. mRNAs are isolated from control and experimental cells or tissues and reverse transcribed in the presence of fluorescently labeled nucleotides (or primers), resulting in labeled cDNAs with different fluorophores (red and green strands) for each sample. Competitive hybridization of the red and green cDNAs to the microarray is proportional to the relative abundance of each mRNA in the two samples. The relative levels of red and green fluorescence are measured by microscopic scanning and are displayed as a single color. Red or orange indicates increased expression in the red (experimental) sample, green or yellow-green indicates lower expression, and yellow indicates equal levels of expression in the control and experiment.
A second major application is in genotyping. Analysis of the human genome (and other organisms) has led to the identification of large numbers of single nucleotide polymorphisms (SNPs), which are single nucleotide substitutions at a specific genetic locus (see the chapter titled The Content of the Genome). Individual SNPs occur at known frequencies, which often differ between populations. The most straightforward examples are where the SNP creates a missense mutation within a gene of interest, such as one involved in the metabolism of a drug. People carrying one allele of the SNP might clear a drug from circulation at a very different rate from those with an alternate allele, and thus determination of a patient’s allele at this SNP can be an important consideration in choosing an appropriate drug dosage. An example of this that has come all the way from theory into everyday use is CYP450 SNP genotyping to determine appropriate dosage of the anticoagulant warfarin. Another is in SNP genotyping of the K-Ras oncogene in some types of cancer patients in order to determine whether EGFR-inhibitory drugs will be of therapeutic value. Other SNPs might be of no direct biological consequence but can become a valuable genetic marker if found to be closely associated to a particular allele of interest—that is, if in genetic terms it is closely linked. Hundreds of thousands of SNPs have been mapped in the human genome, and arrays that can be probed with a subject’s DNA allow for the genotype at each of these to be simultaneously determined, with concurrent determination of what the linked genetic alleles are. In effect, this allows for much of the genotype of the subject to be inferred from a single experiment at vastly less time and expense than actually sequencing the entire subject genome. With a view toward the future, however, it should be noted that SNP genotyping —in the common case of linked alleles as opposed to direct missense mutation alleles—is indirect inference and has at least some potential for being inaccurate.
Sequencing, on the other hand, is definitive. If emerging sequencing technologies improve to the point of offering an entire human genome in 24 hours for a competitive cost to SNP genotyping, it might move to become the dominant approach for genotyping.
A third major application of DNA microarrays is array-comparative genomic hybridization (array-CGH). This is a technique that is augmenting, and in some cases replacing, cytogenetics for the detection and localization of chromosomal abnormalities that change the copy number of a given sequence—that is, deletions or duplications. In this technique, the array chip, known as a tiling array, is spotted with an organism’s genomic sequences that together represent the entire genome; the higher the density of the array, the smaller the genetic region each spot represents and thus the higher resolution the assay can provide. Two DNA samples (one from normal control tissue and one from the tissue of interest) are each randomly labeled with a different fluorophore, such that one sample, for example, is green and the other is red (similar to the mRNA labeling described earlier for the expression arrays).
These two differentially labeled specimens are mixed at exactly equal ratios for total DNA, and then hybridized to the chip. Regions of DNA that occur equally in the two samples will hybridize equally to their complementary array spots, giving a “mixed” color signal.
By comparison, any DNA regions that occur more in one sample than the other will outcompete and thus show a stronger color on their complementary probe spot than will the deficient sample. Computer-assisted image analysis can read out and quantitate small color changes on each array spot and thus detect hemizygous loss or duplication of even very small regions in a test sample. The resolution and facility for automation provided by this technique compared to conventional cytogenetics is leading to its increasing adoption in diagnostic settings for the detection of chromosomal copy number changes associated with a range of hereditary diseases.
Tiling arrays are also often used for chromatin immunoprecipitation studies, which can identify sequences interacting with a DNAbinding protein or complex on a genome-wide scale; this is described in the section Chromatin Immunoprecipitation. In addition to the chip-like solid-phase arrays described, lowerdensity arrays for focused applications (with up to a few hundred
targets, as opposed to millions) can be made in microbead-based formats. In these approaches, each microscopic bead has a distinct optical signal or code, and its surface can be coated with
the target DNA sequence. Different bead codes can be mixed and matched into a single labeled sample of DNA or cDNA and then sorted, detected, and quantitated by optical and/or flow sorting methods. Although of much lower density than chip-type arrays, bead arrays can be modified and adapted much more readily to suit a particular focused biological question, and in practice they show faster three-dimensional hybridization kinetics than chips, which effectively have two-dimensional kinetics.



|
|
|
|
إجراء أول اختبار لدواء "ثوري" يتصدى لعدة أنواع من السرطان
|
|
|
|
|
|
|
دراسة تكشف "سببا غريبا" يعيق نمو الطيور
|
|
|
|
|
|
مجلس أعيان كربلاء يشيد بجهود العتبة العباسية المقدسة في مشروع الحزام الأخضر
|
|
|
|
قسم الشؤون الفكرية يقيم دورة تخصّصية حول الفهرسة الحديثة لملاكات جامعة البصرة
|
|
|
|
الهيأة العليا لإحياء التراث تبحث مع جامعة الكوفة إقامة النشاطات العلميّة المشتركة
|
|
|
|
المجمع العلمي يستأنف الختمة القرآنيّة اليوميّة في الصحن العبّاسي الشريف
|