


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 2-12-2020
Date: 2-12-2020
Date: 2-12-2020
|
Magnetic Nanoparticles
Nanoscaling Laws and Magnetism
Biological examples of magnetic nanoparticles were first discovered in magnetotactic bacteria. These unique iron oxide magnetites are used by these organisms to orient themselves into an optimal environment. Magnetic nanoparticles have also been found in the nasal capsules of salmon. These particles are believed to respond to the geomagnetic field of the Earth, providing the salmon with assistance in reaching their spawning grounds. Similar nanostructures have also been found in the brains of homing pigeons, sea turtles and even humans. Scientists have developed artificial magnetic nanoparticles through chemical synthesis. Recent advances have provided new types of magnetic nanoparticles with precisely tuned compositions, shapes and size. As with many nanostructures, a number of interesting phenomena have been observed in these particles that are distinct from their bulk properties.
In the bulk, the fundamental magnetic properties of coercivity (Hc) and susceptibility (x) are determined principally by the parameters of composition, crystallographic structure, magnetic anisotropy and vacancies and defects. Yet, when magnetic materials are reduced in size to the nanoscale regime, these fundamental properties are no longer permanent.
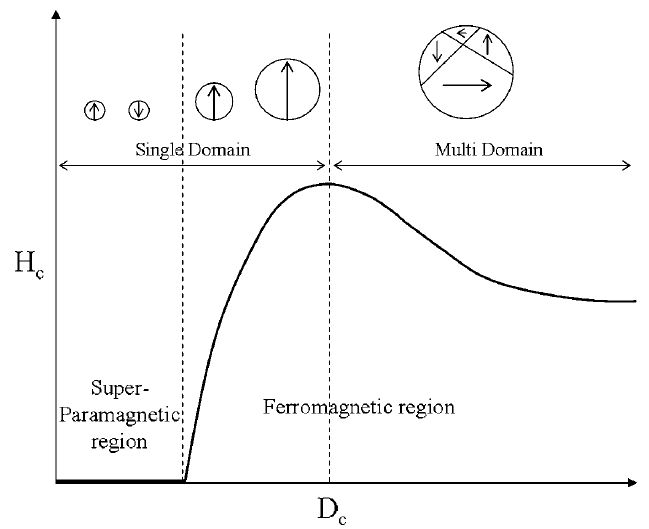
Figure 1 :Size-dependent magnetic domain structures. Spin alignment occurs in single domain particles having sizes below a critical size (Dc). Reproduced by permission of The Royal Society of Chemistry.
Furthermore, the size, shape and compositional structure become important determinants of the magnetic properties. One of the interesting size-dependent phenomena of magnetism on the nanoscale is the observed changes in magnetic coercivity. Whereas bulk magnets contain multiple magnetic domain structures, nanoparticles possess single domain magnetic structures below a specific critical size (Dc). Below this size, all of the magnetic spins in the nanoparticles align unidirectionally (Figure 1). The magnetic coercivity has been shown to increase as the size of the nanoparticles increases with the relationship
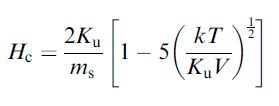
where ms is the saturation magnetization.
The saturation magnetization of the particles themselves is also strongly dependent on the size. In bulk materials, the intrinsically disordered magnetic spin layers near the surface are negligible, since the surface layer is minimal compared with the entire volume of the magnet. Yet, on the nanoscale, the disordered surface effects can be dramatic as they now represent a significantly larger fraction of the total volume This size effect follows the relationship described as
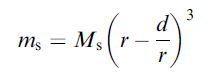
where r is the size, Ms is the saturation magnetization of bulk material and d is the thickness of the disordered surface layer. This effect can be seen in the case of magnetism-engineered iron oxide (MEIO), Fe3O4 nanoparticles. As the size of the MEIO nanoparticles increases over a range 4–12 nm, the mass magnetization values increase linearly as a plot of ms1/3 versus r-1. Such size-dependent properties directly affect their magnetic resonance (MR) signal enhancement capabilities for molecular imaging methodologies.
The crystallinity of the magnetic nanoparticles plays an important role in determining its magnetic coercivity. This is not surprising when one considers how the degree of crystallinity and the organization that the crystal lattice must impart on the interactions of spin within the nanoparticles. This can be seen in magnetic nanoalloys with anisotropic crystalline structures. Co–Pt core–shell nanoparticles composed of an isotropically structured face-centered cubic (fcc) Co core and a nonmagnetic Pt shell displays superparamagnetic behavior with zero coercivity at room temperature. When the particle is annealed, the resulting CoPt nanoalloy adopts a crystalline face-centered tetragonal (fct) structure with room temperature ferromagnetic behavior and a coercivity value of 5300 Oe.
The use of magnetic dopants to modify the composition of nanoparticles can provide access to tunable magnetization. MEIO particles, Fe3O4, have a ferrimagnetic spin structure. In the fcc-packed oxygen lattice, Fe2+ and Fe3+ ions occupying octahedral (Oh) sites have spin that aligns parallel to an external magnetic field (Figure 2), whereas Fe31 ions occupying tetrahedral (Td) sites have spins that align antiparallel to the field. Since high-spin Fe3+ and Fe2+ have a d5 and d6 electron count, respectively, the total magnetic moment per unit (Fe3+)Td (Fe2+Fe3+)OhO4 is approximately 4 μB. Incorporation of an M21 (M¼ Mn, Co, Ni) magnetic dopant with electronic configurations of d5, d4 and d3, respectively, at the Oh Fe2+ site result in a predictable change in the net magnetization to 5, 3 and 2 μB, respectively (Figure 3).
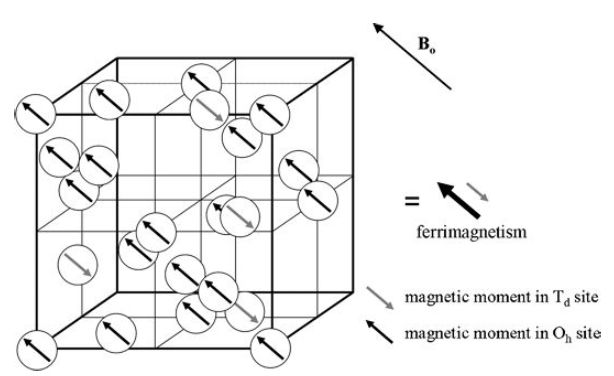
Figure 2. Ferrimagnetic spin structure of fcc-packed Fe3O4 lattices. Reprinted with permission from Jun et al., J. Am. Chem. Soc., 2008, 41, 179. Copyright 2008 American Chemical Society.
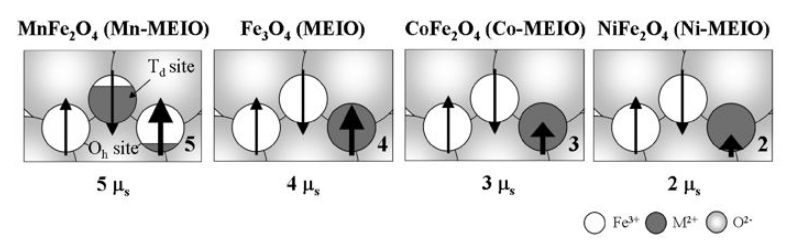
Figure 3. Approximate magnetization values metal-doped MEIO nanoparticles. Reprinted from Nature, 2007, 13, 95, with permission from Macmillan Publishers Ltd.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|