Preparation of enolates
Alkyl hydrogen atoms bonded to a carbon atom at an α (alpha) position relative to a carbonyl group display unusual acidity. While the pKa values for alkyl C-H bonds is typically on the order of 40-50, pKa values for these alpha hydrogens is more on the order of 19-20. This can most easily be explained by resonance stabilization of the product carbanion, as shown:
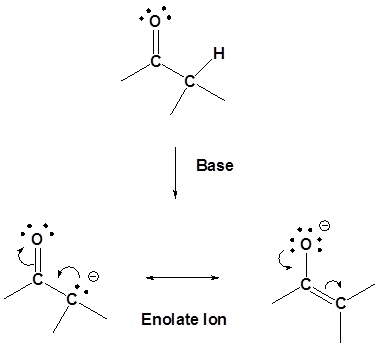
The enolate has two resonance forms – the negative charge can be either on carbon or oxygen – but enolates usually react as nucleophiles from the carbon with enolate alkylation SN2 reactions.
For these alkylation reactions to be useful, the enolate anions must be generated in high concentration in the absence of other strong nucleophiles and bases. Aqueous base (e.g., aq. NaOH) and alkoxides (e.g., NaOCH2CH3) are usually not be suitable because they produce only low concentrations of the enolate anions, and the remaining -OH or -OR can cause unwanted side reactions. In other words, these nucleophilic bases will simply react directly with the alkyl halide via an SN2 reaction.
Some bases that have been successfully used for enolate anion formation are: NaH (sodium hydride, pKa > 45), NaNH2 (sodium amide, pKa = 34), and LiN[CH(CH3)2]2 (lithium diisopropylamide, LDA, pKa 36). Ether solvents like tetrahydrofuran (THF) are commonly used for enolate anion formation.

Because of its solubility in THF, LDA is a widely used base for enolate anion formation. In this application, one equivalent of diisopropylamine is produced along with the lithium enolate, but this normally does not interfere with the enolate reactions and is easily removed from the products by washing with aqueous acid. Many ketones form enolates cleanly with LDA, for example cyclohexanone:

Although the reaction of carbonyl compounds with sodium hydride is slow, sodium enolates are formed with the loss of hydrogen, and no other organic compounds are produced.

Unfortunately, aldehydes do not react cleanly with strong bases to form enolates, so enolate formation with LDA is normally only performed with ketones. However, some other related structures such as esters and nitriles can also form stabilized carbanions with LDA similar to ketone enolates, and these can react in similar ways to ketone enolates:

If the formed enolate is stabilized by more than one carbonyl it is possible to use a weaker base such as sodium ethoxide to form the enolate almost quantitatively.
NaOCH2CH3 = Na+ –OCH2CH3 = NaOEt

Enolates are very useful in synthesis, as they represent a stabilized nucleophilic form of carbon. This chart shows the range of reactions that can be used: