


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-5-2019
Date: 7-2-2018
Date: 29-6-2020
|
When a compound containing carbon and hydrogen is subject to combustion with oxygen in a special combustion apparatus all the carbon is converted to CO2 and the hydrogen to H2O (Figure 1). The amount of carbon produced can be determined by measuring the amount of CO2 produced. This is trapped by the sodium hydroxide, and thus we can monitor the mass of CO2 produced by determining the increase in mass of the CO2 trap. Likewise, we can determine the amount of H produced by the amount of H2O trapped by the magnesium perchlorate.
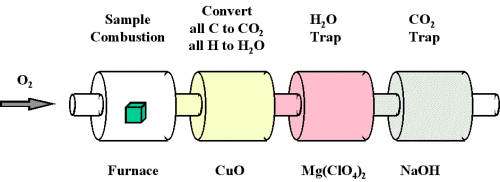
Figure 1 : Combustion analysis apparatus
One of the most common ways to determine the elemental composition of an unknown hydrocarbon is an analytical procedure called combustion analysis. A small, carefully weighed sample of an unknown compound that may contain carbon, hydrogen, nitrogen, and/or sulfur is burned in an oxygen atmosphere,Other elements, such as metals, can be determined by other methods. and the quantities of the resulting gaseous products (CO2, H2O, N2, and SO2, respectively) are determined by one of several possible methods. One procedure used in combustion analysis is outlined schematically in Figure 1 and a typical combustion analysis is illustrated in Examples 1 and 2.
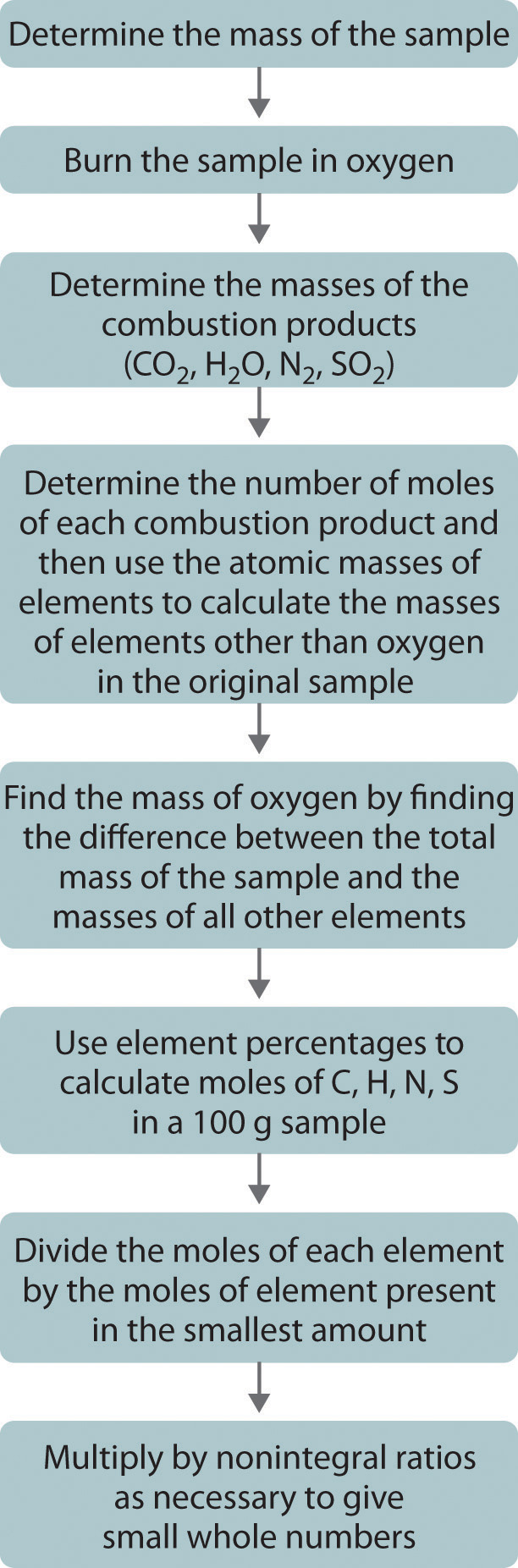
Figure 1: Steps for Obtaining an Empirical Formula from Combustion Analysis. (CC BY-NC-SA; anonymous by request)
Example 1: Combustion of Isopropyl Alcohol
What is the empirical formulate for isopropyl alcohol (which contains only C, H and O) if the combustion of a 0.255 grams isopropyl alcohol sample produces 0.561 grams of CO2 and 0.306 grams of H2O?
Solution
From this information quantitate the amount of C and H in the sample.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|