
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 25-9-2018
Date: 22-9-2018
Date: 19-7-2017
|
ZERO ORDER REACTION
A reactant whose concentration does not affect the reaction rate is not included in the rate law. In effect, the concentration of such a reactant has the power 0. Thus [A] 0 = 1.
A zero order reaction is one whose rate is independent of concentration. For example, the rate law for the reaction at 200° C is
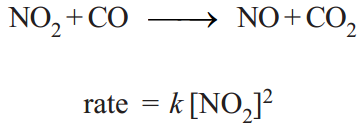
Here the rate does not depend on [CO], so this is not included in the rate law and the power of [CO] is understood to be zero. The reaction is zeroth order with respect to CO. The reaction is second order with respect to [NO2]. The overall reaction order is 2 + 0 = 2.



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|