
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 22-9-2018
Date: 25-9-2018
Date: 19-12-2020
|
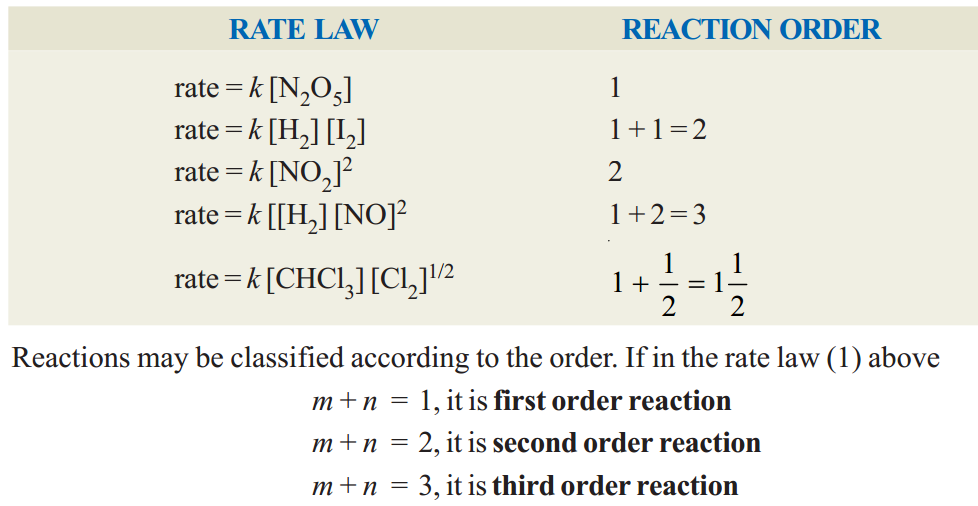
ORDER OF A REACTION
The order of a reaction is defined as the sum of the powers of concentrations in the rate law.
Let us consider the example of a reaction which has the rate law
rate = k[A]m[B]n ...(1)
The order of such a reaction is (m+ n).
The order of a reaction can also be defined with respect to a single reactant. Thus the reaction order with respect to A is mand with respect to B it is n. The overall order of reaction (m+ n) may range from 1 to 3 and can be fractional.
Examples of reaction order :



|
|
|
|
دور في الحماية من السرطان.. يجب تناول لبن الزبادي يوميا
|
|
|
|
|
|
|
العلماء الروس يطورون مسيرة لمراقبة حرائق الغابات
|
|
|
|
|
|
|
ضمن أسبوع الإرشاد النفسي.. جامعة العميد تُقيم أنشطةً ثقافية وتطويرية لطلبتها
|
|
|