


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-10-2019
Date: 14-10-2019
Date: 3-11-2019
|
The Mannich reaction is similar to a crossed aldol reaction in that a nucleophilic enol-donor forms a carbon-carbon bond to an electrophilic iminium-acceptor. This assembly is outlined at the top of the following diagram, using a methyl ketone as the enol precursor and an aldehyde iminium electrophile. Formaldehyde is a commonly used aldehyde reactant, as shown in the example below the mechanism. The Mannich base product is a β-aminoketone, usually formed as a mineral acid salt. These bases find use as stable precursors of reactive α,β-unsaturated carbonyl compounds, as illustrated in the same example. Eschenmoser's salt is a particularly useful source of formaldehyde for Mannich reactions.
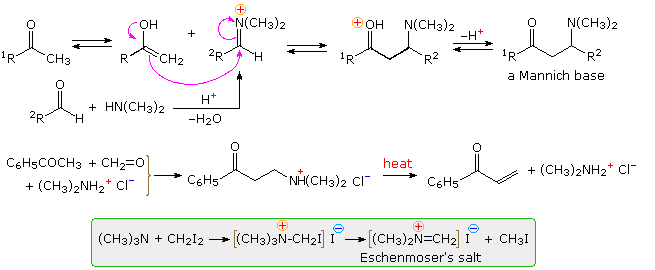
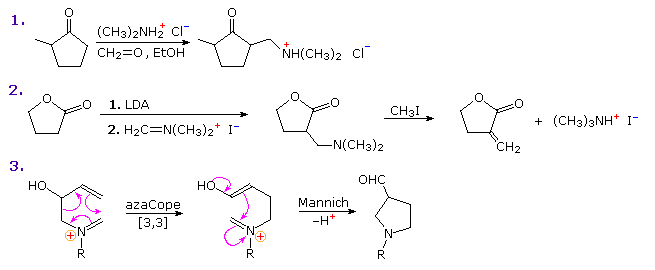
The first is a straightforward reaction involving the kinetically favored enol of a cyclic ketone. The second shows the reaction of Eschenmoser's salt with a preformed lithium enolate. Such reactions produce the free base directly. In order to effect the β-elimination of the amine, it is first converted to a quaternary ammonium salt by methylation. Subsequent elimination is very rapid. The last example is an unconventional case in which an intramolecular Mannich precursor is generated in situ by an azaCope rearrangement.
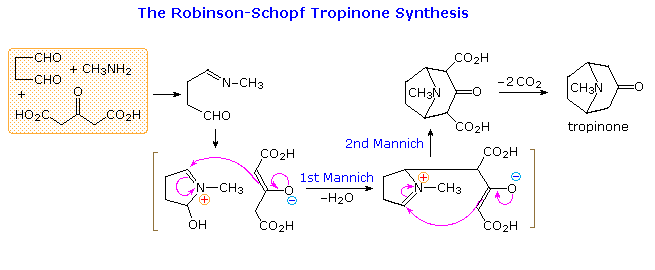
By clicking on the diagram a second time, a remarkable synthesis of the bicyclic aminoketone tropinone will appear above. This double Mannich sequence is known as the Robinson-Schopf synthesis in honor of the two chemists who first reported it in the first quarter of the 20th century. The reaction takes place in water under physiological conditions (pH and temperature), and in high yield.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|