


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 30-3-2019
Date: 15-10-2018
Date: 5-12-2018
|
Diagonal relationships between Li and Mg, and between Be and Al
Li and its compounds are often considered to be anomalous when compared with those of the later group 1 metals, and that a diagonal relationship exists between Li and Mg. So we consider this relationship in detail and also describe a similar diagonal relationship between Be and Al. The positions of Li, Be, Mg and Al in the periodic table are shown in below.
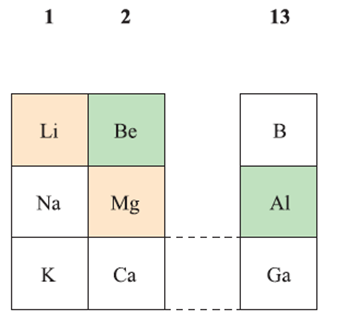
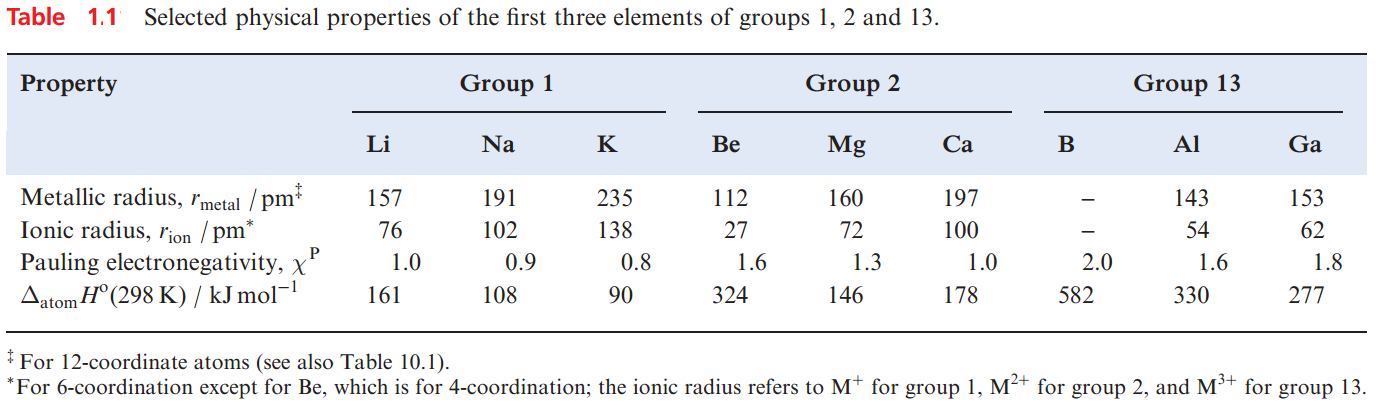
Table 1.1 lists selected physical properties of the first three elements in groups 1, 2 and 13. From a comparison of the properties of Li with those of Na and K, or of Li with Mg, it can be seen that Li resembles Mg more closely than it does the later members of group 1. A similar comparison between Be, Mg, Ca and Al leads to the conclusion that the physical properties of Be listed in Table 1.1 resemble those of Al more than they do those of the later group 2 metals.
One crucial factor is that the charge densities of Li and Mg2+ are similar because the increase in charge is offset by an increase in ion size. Likewise, the charge densities of Be2+ and Al3+ are similar.
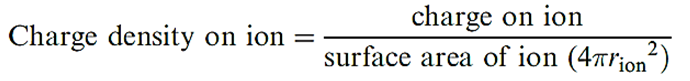
These diagonal relationships result in similarities between the chemistries of Li and Mg, and between Be and Al, and set the first members of each group apart from their heavier congeners. Small cations such as Li, Mg2+ , Be2+ and Al3+ possess high charge densities and each has a high polarizing power.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|