


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 30-3-2019
Date: 22-11-2018
Date: 30-3-2019
|
The diagram below shows how atomic radius changes across Period 3.
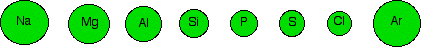
The figures used to construct this diagram are based on:
It is appropriate to compare metallic and covalent radii because they are both being measured in tightly bonded circumstances. These radii cannot be compared with a van der Waals radius, however, making the diagram deceptive. The general trend towards smaller atoms across the period is not broken at argon. For convenience and clarity, argon is ignored in this discussion.
A metallic or covalent radius is a measure of the distance from the nucleus to the bonding pair of electrons. From sodium to chlorine, the bonding electrons are all in the 3-level, screened by the electrons in the first and second levels. The increasing number of protons in the nucleus across the period attracts the bonding electrons more strongly. The amount of screening is constant across Period 3.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|