


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 21-1-2016
Date: 26-1-2020
Date: 20-9-2017
|
POLYPROPYLENE
Polypropylene (PP) is a major thermoplastic polymer. Although polypropylene did not take its position among the large volume polymers until fairly recently, it is currently the third largest thermoplastic after PVC. The delay in polypropylene development may be attributed to technical reasons related to its polymerization. Polypropylene produced by free radical initiation is mainly the atactic form. Due to its low crystallinity, it is not suitable for thermoplastic or fiber use. The turning point in polypropylene production was the development of a Ziegler-type catalyst by Natta to produce the stereoregular form (isotactic).
Catalysts developed in the titanium-aluminum alkyl family are highly reactive and stereoselective. Very small amounts of the catalyst are needed to achieve polymerization (one gram catalyst/300,000 grams polymer). Consequently, the catalyst entrained in the polymer is very small, and the catalyst removal step is eliminated in many new processes.
Amoco has introduced a new gas-phase process called “absolute gasphase” in which polymerization of olefins (ethylene, propylene) occurs in the total absence of inert solvents such as liquefied propylene in the reactor. Titanium residues resulting from the catalyst are less than 1 ppm, and aluminum residues are less than those from previous catalysts used in this application.
Polypropylene could be produced in a liquid or in a gas-phase process. Until 1980, the vertically stirred bed process of BASF was the only largescale commercial gas phase process. In the Union Carbide/Shell gas phase process (Figure 1.1), a wide range of polypropylenes are made in a fluidized bed gas phase reactor.
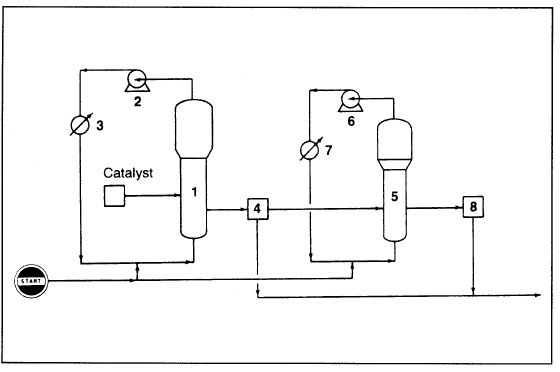
Figure 1.1. The Union Carbide gas-phase process for producing polypropylene: (1) reactor, (2) centrifugal compressor, (3) heat exchanger, (4) product discharge tank (unreacted gas separated from product), (5) impact reactor, (6) compressor, (7) heat exchanger, (8) discharge tank (copolymer separated from reacted gas).
Melt index, atactic level, and molecular weight distribution are controlled by selecting the proper catalyst, adjusting operating conditions, and adding molecular weight control agents. This process is a modification of the polyethylene process (discussed before), but a second reactor is added. Homopolymers and random copolymers are produced in the first reactor, which operates at approximately 70°C and 35 atmospheres. Impact copolymers are produced in the second reactor (impact reactor) after transferring the polypropylene resin from the first reactor. Gaseous propylene and ethylene are fed to the impact reactor to produce the polymers’ rubber phase.
Operation of the impact reactor is similar to the initial one, but the second operates at lower pressure (approximately 17 atmospheres). The granular product is finally pelletized. Random copolymers made by copolymerizing equal amounts of ethylene and propylene are highly amorphous, and they have rubbery properties.
An example of the liquid-phase polymerization is the Spheripol process (Figure 1.2), which uses a tubular reactor.
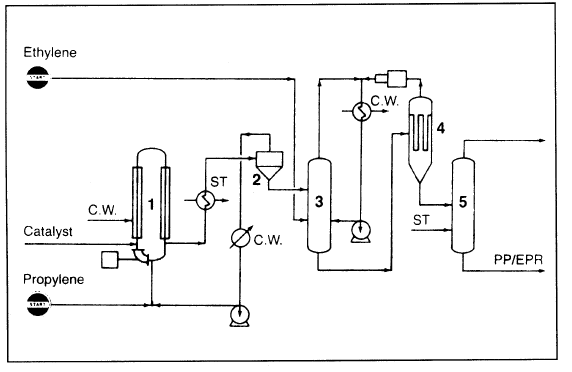
Figure 1.2. The Himont Inc. Spheripol process for producing polypropylene in a liquid-phase: (1) tubular reactor, (2,4) two-stage flash pressure system (to separate unreacted monomer for recycle), (3) heterophasic copolymerization gasphase reactor, (5) stripper.
Copolymerization occurs in a second gas phase reactor. Unreacted monomer is flashed in a two-stage pressure system and is recycled back to the reactor. Polymer yields of 30,000 or more Kg/Kg of supported catalyst are attainable, and catalyst residue removal from the polymer is not required. The product polymer has an isotactic index of 90–99%.
New generation metallocene catalysts can polymerize propylene in two different ways. Rigid chiral metallocene produce isotactic polypropylene whereas the achiral forms of the catalysts produce atactic polypropylene. The polymer microstructure is a function of the reaction conditions and the catalyst design. Recent work has shown that the rate of ligand rotation in some unbridged metallocenes can be controlled so that the metallocene oscillates between two stereochemical states. One isomer produces isotactic polypropylene and the other produces the atactic polymer. As a result, alternating blocks of rigid isotactic and flexible atactic polypropylene grow within the same polymer chain.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|