


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 20-9-2017
Date: 29-9-2017
Date: 20-9-2017
|
POLYETHYLENE
Polyethylene is the most extensively used thermoplastic. The everincreasing demand for polyethylene is partly due to the availability of the monomer from abundant raw materials (associated gas, LPG, naphtha). Other factors are its relatively low cost, ease of processing the polymer, resistance to chemicals, and its flexibility. World production of all polyethylene grades, approximately 100 billion pounds in 1997, is predicted to reach 300 billion pounds in 2015, the largest increase for linear low density polyethylene.
High-pressure polymerization of ethylene was introduced in the 1930s. The discovery of a new titanium catalyst by Karl Ziegler in 1953 revolutionized the production of linear unbranched polyethylene at lower pressures.
The two most widely used grades of polyethylene are low-density polyethylene (LDPE) and high-density polyethylene (HDPE). Currently, a new LDPE grade has been introduced. It is a linear, low-density grade (LLDPE) produced like the high-density polymer at low pressures. Polymerizing ethylene is a highly exothermic reaction. For each gram of ethylene consumed, approximately 3.5 KJ (850 cal) are released:
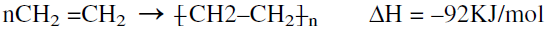
When ethylene is polymerized, the reactor temperature should be well controlled to avoid the endothermic decomposition of ethylene to carbon, methane, and hydrogen:
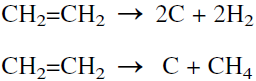



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|