
 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 7-9-2019
Date: 25-8-2019
Date: 30-6-2018
|
The relative reactivity of the carboxylic acid derivatives is an important concept to understand before entering into a detailed examination of nucleophilic acyl substitutions. As a general rule, the carbonyl carbon in an acyl group is less electrophilic than that in an aldehyde or ketone. This is because in carboxylic acid derivatives, the partial positive charge on the carbon is stabilized somewhat by resonance effects from the adjacent heteroatom.
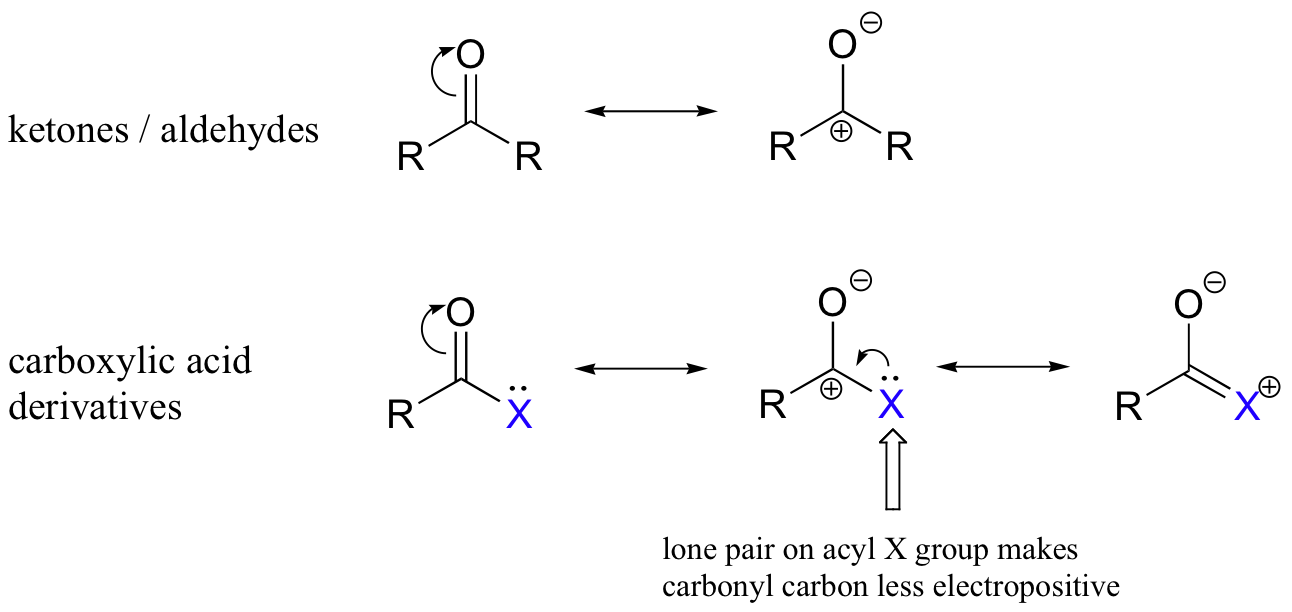
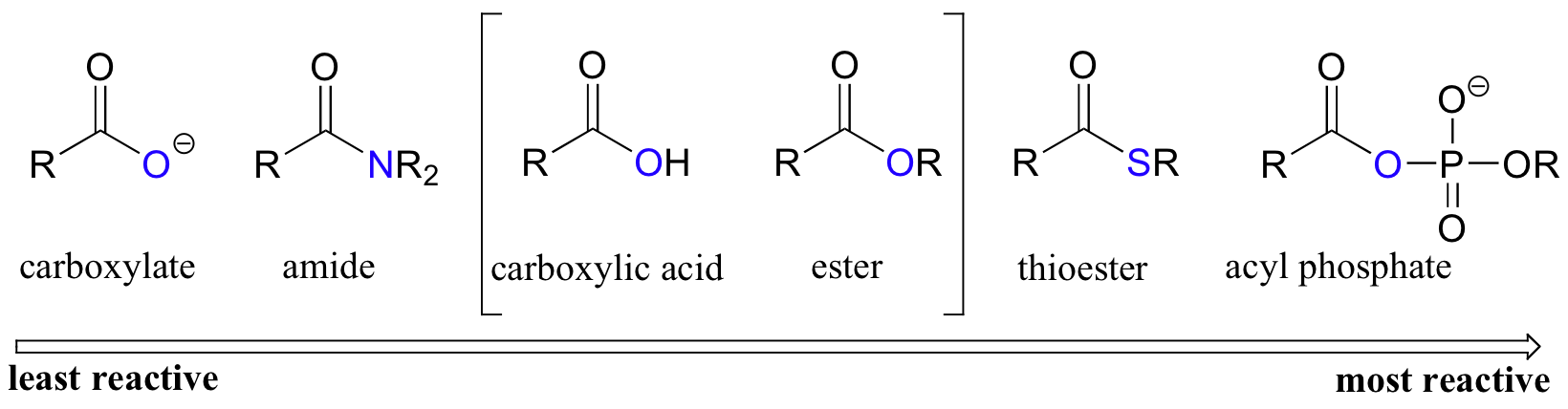
Among the carboxylic acid derivatives, carboxylate groups are the least reactive towards nucleophilic acyl substitution, followed by amides, then esters and (protonated) carboxylic acids, thioesters, and finally acyl phosphates, which are the most reactive among the biologically relevant acyl groups.
The different reactivities of the functional groups can be understood by evaluating the basicity of the leaving group in each case - remember from section 8.5 that weaker bases are better leaving groups! A thioester is more reactive than an ester, for example, because a thiolate (RS-) is a weaker base than an alkoxide (RO-). In general, if the incoming nucleophile is a weaker base than the ‘acyl X’ group that is already there, the first nucleophilic step will simply reverse itself and we’ll get the starting materials back:
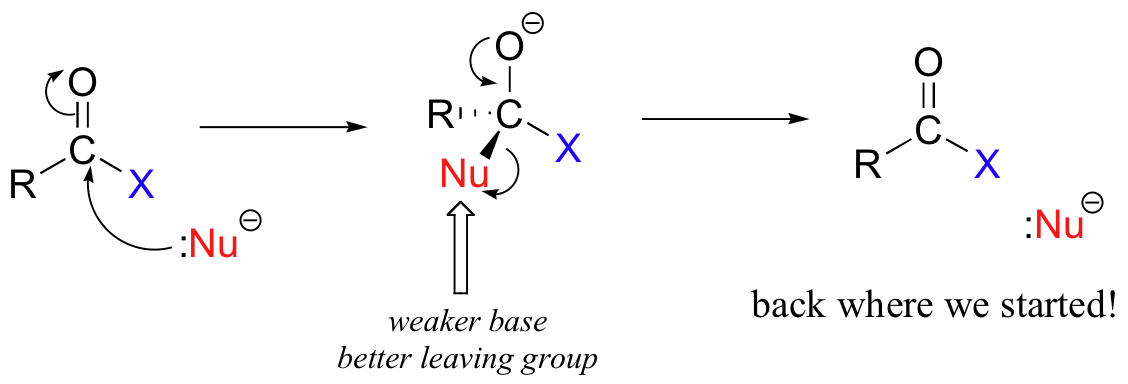
This is why it is not possible to directly convert an ester, for example, into a thioester by an acyl substitution reaction – this would be an uphill reaction.
Here’s another way to think about the relative reactivites of the different carboxylic acid derivatives: consider the relative electrophilicity, or degree of partial positive charge, on the carbonyl carbon in each species. This depends on how much electron density the neighboring heteroatom on the acyl X group is able to donate: greater electron donation by the heteroatom implies lower partial positive charge on the carbonyl carbon, which in turn implies lower electrophilicity.
The negatively charged oxygen on the carboxylate group has lots of electron density to donate, thus the carbonyl carbon is not very electrophilic. In amides, the nitrogen atom is a powerful electron donating group by resonance - recall that the carbon-nitrogen bond in peptides has substantial double-bond character - thus amides are relatively unreactive. Amides do undergo acyl substitution reactions in biochemical pathways, but these reactions are inherently slow and the enzymes catalyzing them have evolved efficient strategies to lower the activation energy barrier.
Carboxylic acids and esters are in the middle range of reactivity, while thioesters are somewhat more reactive. The most reactive of the carboxylic acid derivatives frequently found in biomolecules are the acyl phosphates. These are most often present in two forms: the simple acyl monophosphate, and the acyl-adenosine monophosphate.
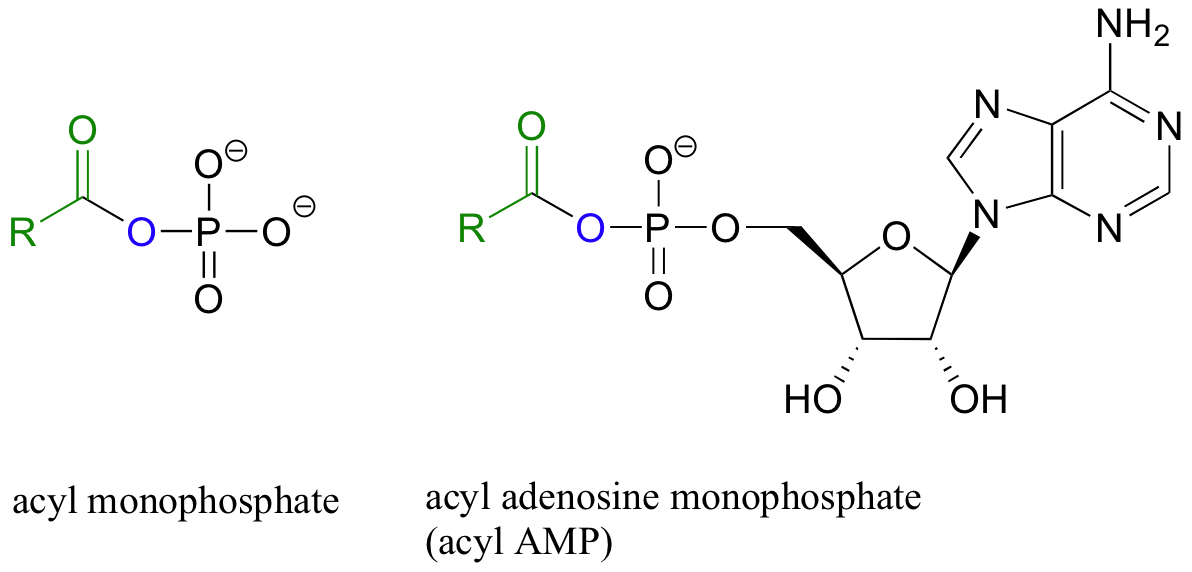
Both are highly reactive to acyl substitution reactions, and are often referred to as ‘activated acyl groups’ or ‘activated carboxylic acids’. The high reactivity of acyl phosphates is due mainly to the ability to form complexes with magnesium ions.
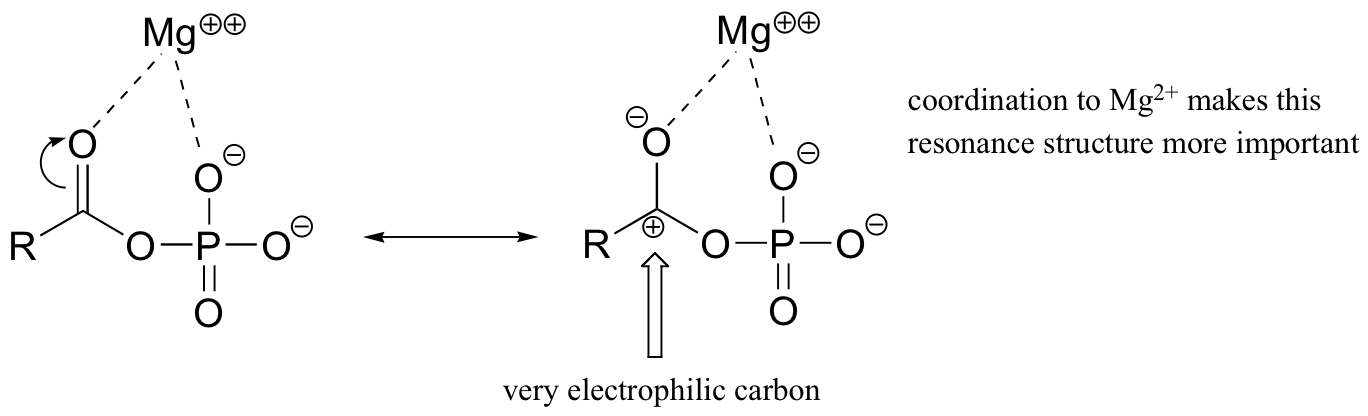
The magnesium ion acts as a Lewis acid to accept electron density from the oxygen end of the acyl carbonyl bond, thus greatly increasing the degree of partial positive charge - and thus the electrophilicity - of the carbonyl carbon. The magnesium ion also balances negative charge on the phosphate, making it an excellent leaving group.
In our examination of acyl substitution reactions, we will start with the formation and reactions of the highly reactive acyl phosphates. We will then discuss how thioesters play a key role in the acyl substitution reactions of lipid metabolism. Finally, we will take a look at some important acyl substitution reactions involving esters, as well as the formation and cleavage of the amide linkages in the peptide bonds of proteins.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
قسم الشؤون الفكرية والثقافية يجري اختبارات مسابقة حفظ دعاء أهل الثغور
|
|
|