

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Biological Reduction of Aldehydes and Ketones
المؤلف:
..................
المصدر:
LibreTexts Project
الجزء والصفحة:
.................
7-9-2019
1578
Biological Reduction of Aldehydes and Ketones
Addition to a carbonyl by a semi-anionic hydride, such as NaBH4, results in conversion of the carbonyl compound to an alcohol. The hydride from the BH4- anion acts as a nucleophile, adding H- to the carbonyl carbon. A proton source can then protonate the oxygen of the resulting alkoxide ion, forming an alcohol.
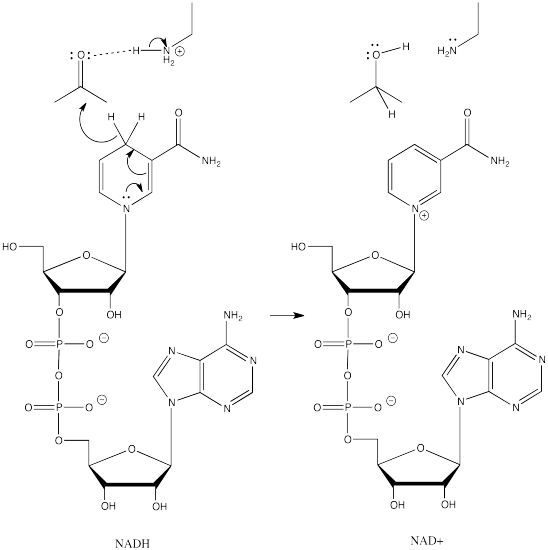
Formally, that process is referred to as a reduction. Reduction generally means a reaction in which electrons are added to a compound; the compound that gains electrons is said to be reduced. Because hydride can be thought of as a proton plus two electrons, we can think of conversion of a ketone or an aldehyde to an alcohol as a two-electron reduction. An aldehyde plus two electrons and two protons becomes an alcohol.
Aldehydes, ketones and alcohols are very common features in biological molecules. Converting between these compounds is a frequent event in many biological pathways. However, semi-anionic compounds like sodium borohydride don't exist in the cell. Instead, a number of biological hydride donors play a similar role.
NADH is a common biological reducing agent. NADH is an acronym for nicotinamide adenine dinucleotide hydride. Insetad of an anionic donor that provides a hydride to a carbonyl, NADH is actually a neutral donor. It supplies a hydride to the carbonyl under very specific circumstances. In doing so, it forms a cation, NAD+. However, NAD+ is stabilized by the fact that its nicotinamide ring is aromatic; it was not aromatic in NADH.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















