
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 22-8-2017
Date: 24-7-2017
Date: 31-8-2017
|
CARBON DISULFIDE (CS2)
Methane reacts with sulfur (an active nonmetal element of group 6A) at high temperatures to produce carbon disulfide. The reaction is endothermic, and an activation energy of approximately 160 KJ is required. Activated alumina or clay is used as the catalyst at approximately 675°C and 2 atmospheres. The process starts by vaporizing pure sulfur, mixing it with methane, and passing the mixture over the alumina catalyst. The reaction could be represented as:
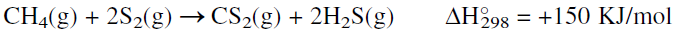
Hydrogen sulfide, a coproduct, is used to recover sulfur by the Claus reaction. A CS2 yield of 85–90% based on methane is anticipated. An alternative route for CS2 is by the reaction of liquid sulfur with charcoal. However, this method is not used very much.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|