


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 21-9-2020
Date: 8-10-2018
Date: 8-10-2018
|
Hydration of Alkenes: Addition of H2O by Oxymercuration
Water adds to alkenes to yield alcohols, a process called hydration. This reaction takes place on treatment of the alkene with water and a strong acid catalyst, such as H2SO4, by a mechanism similar to that of HX addition. Thus, as shown in Figure 1.1, protonation of an alkene double bond yields a carbocation intermediate, which reacts with water to yield a protonated alcohol product, ROH2+. Loss of H+ from this protonated alcohol gives the neutral alcohol and regenerates the acid catalyst.
Figure 1.1
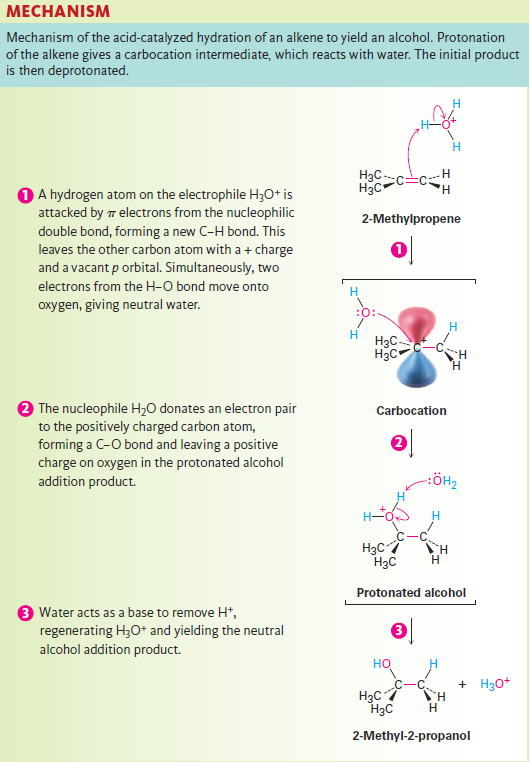
Acid-catalyzed alkene hydration is particularly suited to large-scale industrial procedures, and approximately 300,000 tons of ethanol are manufactured each year in the United States by hydration of ethylene. The reaction is of little value in the typical laboratory, however, because it requires high temperatures—250 °C in the case of ethylene—and strongly acidic conditions.

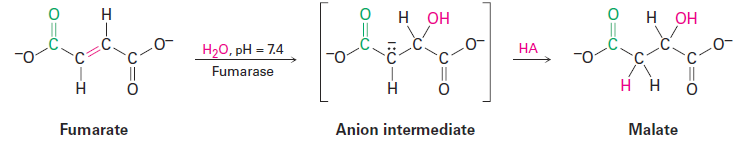
Acid-catalyzed hydration of isolated double bonds, although known, is also uncommon in biological pathways. More frequently, biological hydrations require that the double bond be adjacent to a carbonyl group for reaction to proceed. Fumarate, for instance, is hydrated to give malate as one step in the citric acid cycle of food metabolism but might note for now that the reaction is not an electrophilic addition but instead occurs through a mechanism that involves formation of an anion intermediate followed by protonation by an acid HA.
When it comes to circumventing problems like those with acid-catalyzed alkene hydrations, laboratory chemists have a great advantage over the cellular “chemists” in living organisms. Laboratory chemists are not constrained to carry out their reactions in water solution; they can choose from any of a large number of solvents. Laboratory reactions don’t need to be carried out at a fixed temperature; they can take place over a wide range of temperatures. And laboratory reagents aren’t limited to containing carbon, oxygen, nitrogen, and a few other elements; they can contain any element in the periodic table.
In the laboratory, alkenes are often hydrated by the oxymercuration– demercuration procedure. Oxymercuration involves electrophilic addition of Hg2+ to the alkene on reaction with mercury(II) acetate [(CH3CO2)2Hg, often abbreviated Hg(OAc)2] in aqueous tetrahydrofuran (THF) solvent. The intermediate organomercury compound is then treated with sodium borohydride, NaBH4, and demercuration occurs to produce an alcohol. For example:
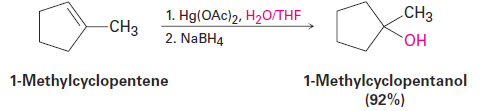
Alkene oxymercuration is closely analogous to halohydrin formation. The reaction is initiated by electrophilic addition of Hg21 (mercuric) ion to the alkene to give an intermediate mercurinium ion, whose structure resembles that of a bromonium ion (Figure 1.2). Nucleophilic addition of water as in halohydrin formation, followed by the loss of a proton, then yields a stable organomercury product. The final step, demercuration of the organomercury compound by reaction with sodium borohydride, is complex and involves radicals.
Note that the regiochemistry of the reaction corresponds to Markovnikov addition of water; that is, the -OH group attaches to the more highly substituted carbon atom, and the -H attaches to the less highly substituted carbon. The hydrogen that replaces mercury in the demercuration step can attach from either side of the molecule depending on the exact circumstances.
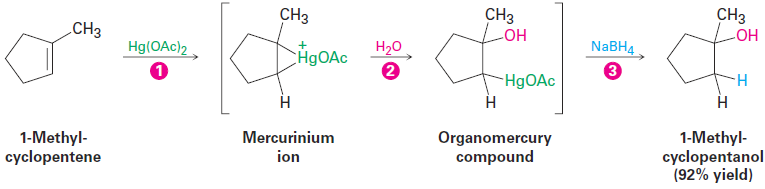
Figure 1.2 Mechanism of the oxymercuration of an alkene to yield an alcohol. ( 1 ) Electrophilic addition of Hg2+ gives a mercurinium ion, which ( 2 ) reacts with water as in halohydrin formation. Loss of a proton gives an organomercury product, and ( 3 ) reaction with NaBH4 removes the mercury. The product of the reaction is a more highly substituted alcohol, corresponding to Markovnikov regiochemistry.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|