


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 28-9-2020
Date: 18-5-2016
Date: 13-12-2020
|
A Closer Look at Heat and Work
When discussing work and energy for thermodynamic systems it is useful to think about compressing the gas in a piston, as shown in Fig. 1.1.

FIGURE 1.1 Piston.
By pushing on the piston the gas is compressed, or if the gas is heated the piston expands. Such pistons are crucial to the operation of automobile engines. The gas consists of a mixture of gasoline which is compressed by the piston. Sitting inside the chamber is a spark plug which ignites the gas and pushes the piston out. The piston is connected to a crankshaft connecting the auto engine to the wheels of the automobile. Another such piston system is the simple bicycle pump. Recall our definition of Work as
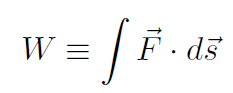
For the piston, all the motion occurs in 1-dimension so that
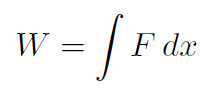
(or equivalently 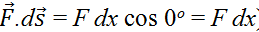 ). The pressure of a gas is defined as force divided by area (of the piston compressing the gas) or
). The pressure of a gas is defined as force divided by area (of the piston compressing the gas) or
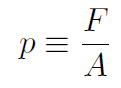
giving dW = pAdx = pdV where the volume is just area times distance or dV = Adx. That is when we compress the piston by a distance dx, the volume of the gas changes by dV = Adx where A is the cross-sectional area of the piston. Writing W = ∫dW gives
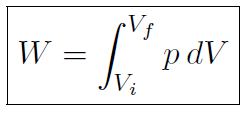
which is the work done by a gas of pressure p changing its volume from Vi to Vf (or the work done on the gas).



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|