


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية | Evidence for metal–ligand covalent bonding: The nephelauxetic effect |
|
|
|
Read More
Date: 23-2-2017
Date: 28-11-2016
Date: 14-9-2016
|
Evidence for metal–ligand covalent bonding: The nephelauxetic effect
In metal complexes, there is evidence for sharing of electrons between metal and ligand. Pairing energies are lower in complexes than in gaseous Mn ions, indicating that interelectronic repulsion is less in complexes and that the effective size of the metal orbitals has increased; this is the nephelauxetic effect.
Table 1.1 Selected values of h and k which are used to parameterize the nephelauxetic series; worked examplebelow shows their application.

For complexes with a common metal ion, it is found that the nephelauxetic effect of ligands varies according to a series independent of metal ion:

A nephelauxetic series for metal ions (independent of ligands) is as follows:
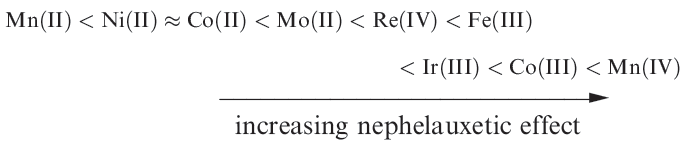
The nephelauxetic effect can be parameterized and the values shown in Table 1.1 used to estimate the reduction in electron–electron repulsion upon complex formation. In equation 1.1, the interelectronic repulsion in the complex is the Racah parameter B . B0 is the interelectronic repulsion in the gaseous Mn ion.
 (1.1)
(1.1)
The worked example and exercises below illustrate how to apply equation 1.1.
Worked example The nephelauxetic series
Using data in Table 1.1, estimate the reduction in the interelectronic repulsion in going from the gaseous Fe3+ ion to [FeF6]3-. The reduction in interelectronic repulsion is given by:

In Table 1.1, values of h refer to an octahedral set of ligands.
 Therefore, the reduction in interelectronic repulsion in going from the gaseous Fe3+ ion to [FeF6]3- is ≈19%.
Therefore, the reduction in interelectronic repulsion in going from the gaseous Fe3+ ion to [FeF6]3- is ≈19%.



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
العتبة العباسية المقدسة تطلق النسخة الحادية عشرة من مسابقة الجود العالمية للقصيدة العمودية
|
|
|