


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 25-2-2018
Date: 24-6-2017
Date: 25-2-2018
|
Molar Concentration
The molar concentration cx of a solution of a solute species X is the number of moles of that species that is contained in 1 liter of the solution (not 1 L of the solvent). In terms of the number of moles of solute, n, and the volume, V, of solution, we write
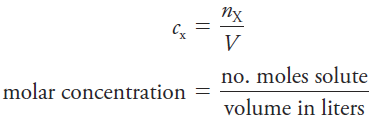
The unit of molar concentration is molar, symbolized by M, which has the dimensions of mol/L, or mol L-1. Molar concentration is also the number of millimoles of solute per milliliter of solution.
Example: Calculate the molar concentration of ethanol in an aqueous solution that contains
2.30 g of C2H5OH (46.07 g/mol) in 3.50 L of solution.
Solution
To calculate molar concentration, we must find both the amount of ethanol and the volume of the solution. The volume is given as 3.50 L, so all we need to do is convert the mass of ethanol to the corresponding amount of ethanol in moles.
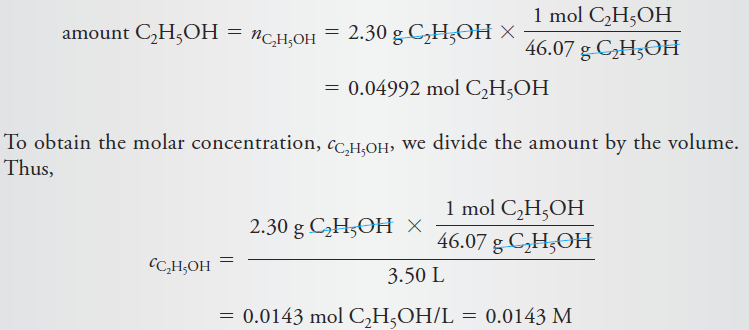



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
مكتب السيد السيستاني يعزي أهالي الأحساء بوفاة العلامة الشيخ جواد الدندن
|
|
|